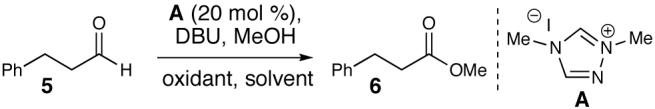
Table 1.
Oxidation Optimization
 | |||
|---|---|---|---|
| entry | oxidant | solvent | yieldb |
| 1 | o-chloranil (2 equiv) | THF | (<10) |
| 2 | TEMPO (1 mol %), NaOCl (aq.) (2 equiv) | CH2Cl2/H2O | 0 |
| 3 | TEMPO (1 mol %), Oxone (4 equiv) | CH2Cl2 | (52) |
| 4 | TEMPO (1 mol %), n-Bu4NSO5c (2 equiv) | CH2Cl2 | (59) |
| 5 | MnO2 (5 equiv) | MeOH | 99 |
| 6 | MnO2 | CH2Cl2 | 98d |
| 7 | MnO2 19 mmol scale | CH2Cl2 | 98d |
20 mol% A, 1.2 equiv DBU, 2-5 equiv methanol, 0.2 M in solvent.
Isolated yields (yields in parentheses calculated by GC with dodecane as an internal standard).
Prepared from commercial Oxone (see Supporting Information).
10 mol% A, 1.1 equiv DBU, 5 equiv methanol, 5 equiv MnO2, 0.2 M in CH2Cl2.
