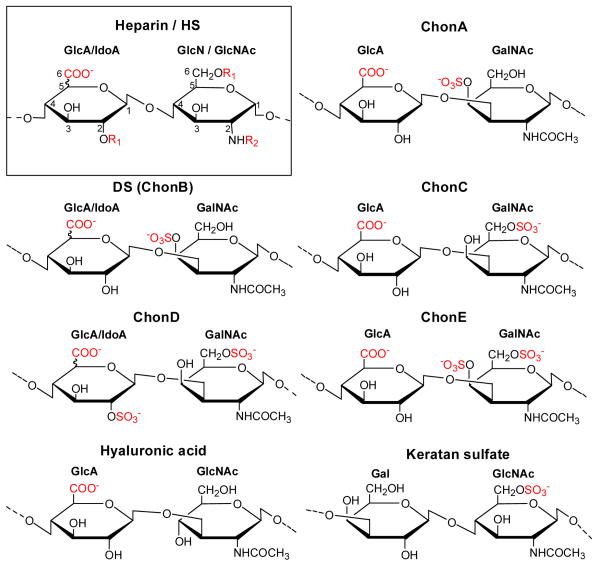
Figure 1.
Heparan sulfate (HS) and heparin have the same repeating disaccharide subunits of glucuronic acid (GlcA) or iduronic acid (IdoA) and glucosamine (GlcN) or N-acetylated glucosamine (GlcNAc) linked by an α-1,4 glycosidic linkage with various sulfation patterns (box). IdoA is formed by epimerization of GlcA at the C5 position. In both heparin and HS, these subunits can have variable N-acetylation and N- or O- sulfation at C2 or C6 positions. HS has fewer sulfates, is less negatively charged, and has a more heterogeneous structure than heparin. Chondroitin sulfate A (ChonA) contains 4-O-sulfo GalNAc; dermatan sulfate (DS) also known as chondroitin sulfate B (ChonB) contains IdoA, 4-O-sulfo GalNAc; chondroitin sulfate C (ChonC) contains 6-O-sulfo GalNAc; chondroitin sulfate D (ChonD) contains 2-O-sulfo IdoA and 6-O-sulfo GalNAc; chondroitin sulfate E (ChonE) contains 4, 6-O-sulfo GalNAc. Hyaluronic acid is a copolymer of GlcA and GlcNAc and is the only GAG that is not sulfated and is not linked to a core protein Keratan sulfate contains galactose units (Gal) instead of hexuronic acid units. R1 indicates that the substituent can be either a hydrogen (-H) or a sulfo group (-SO3-). R2 indicates that the the substituent can be either a hydrogen (-H), a sulfo group (-SO3-) or an acetyl group (-COCH3). Negatively charged groups are shown in red (-COO-, SO3-).