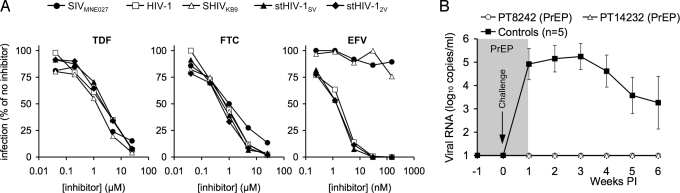
Fig. 5.
Antiretroviral drug prophylaxis of stHIV-1 infection. (A) Sensitivity of SIVMNE027, SHIVKB9, HIV-1, and stHIV-1 strains to inhibition by the nucleoside (TDF and FTC) and non-nucleoside (EFV) RT inhibitors, that comprise a mixture commonly used for treatment of HIV-1 infection. TZM cells were infected with each virus in the presence of the indicated concentrations of each drug, and the level of infection is plotted as the percentage of that in the absence of drug. (B) Two macaques received chemoprophylaxis with a TDF/FTC/EFV combination (PrEP) and were challenged intravenously, at 1 week after prophylaxis initiation, with the same dose of stHIV-1SV/2V as was used in Fig. 2. Treatment was continued for 1 week following the challenge. A contemporaneous control macaque was challenged but untreated. Plasma viremia was measured for the ensuing 5 weeks, using RT-PCR as in Fig. 2, and compared to the 4 previously inoculated and the contemporaneous control for which the mean and standard deviation of the plasma viremia over the first 6 weeks of infection is plotted.