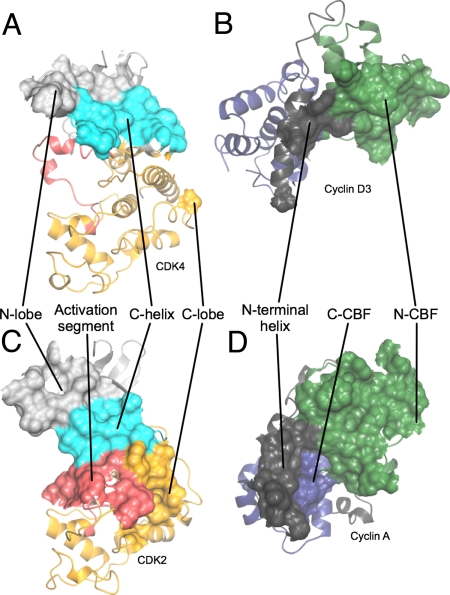
Fig. 3.
Comparison of interfaces involved in the structure of CDK4/cyclin D3 with those involved in the T160pCDK2/cyclin A structure. (A and B) CDK4/cyclin D3. (C and D) T160pCDK2/cyclin A. Shown are the origins of residues on each subunit that compose the CDK/cyclin interface. Peeling the 2 molecules apart reveals that the CDK2/cyclin A and CDK4/cyclin D3 interfaces are very different. Whereas the CDK2/cyclin A interface is extensive and involves residues from both the CDK2 and cyclin A N- and C-terminal folds (C and D), the CDK2 C-helix (C) and activation segment (C) and the cyclin A N-terminal helix (D), that of CDK4/cyclin D3 (A and B) is much more confined and involves a more limited set of residues from the CDK4 and cyclin D3 N-terminal folds and the cyclin D3 N-terminal helix. The CDK N- and C-terminal folds are colored light gray and yellow, respectively, with the C-helix in cyan, and the activation segment in red. The cyclin N- and C- terminal CBFs are colored green and blue, respectively, and the N-terminal helix is colored gray and the C-terminal is helix dark gray. T160pCDK2/cyclin A is PDB code 1QMZ.