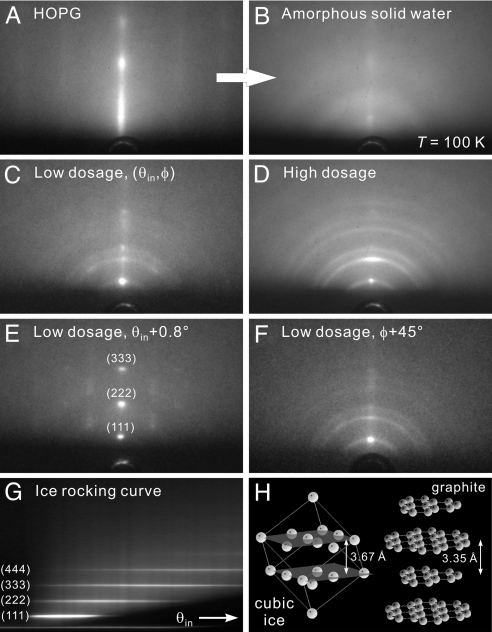
Fig. 1.
Diffraction patterns of ice on hydrophobic graphite at various conditions. (A) Diffractio pattern of a freshly cleaved HOPG surface. The grazing incidence and nanometer depth of electron probing give rise to the rod-like pattern. (B) Diffraction pattern at the same probing condition as A after the deposition of water molecules at a surface temperature of T = 100 K. The diffuse scattering without distinct diffraction features indicates that the water assembly is in an amorphous state. (C) Diffraction pattern from a thin interfacial water layer after its crystallization into an ice assembly through thermal annealing. The most distinct diffraction feature is the intense Bragg spot at the center, signifying an ordered structure along the surface normal direction. (D) The pattern from a thicker ice assembly at the same probing condition as C. Although the central Bragg spot is still apparent, a Debye–Scherrer ring pattern becomes clearly evident, indicating that the ice crystallites away from the substrate surface are randomly oriented. (E) The pattern from a thin ice assembly at a larger incidence angle (θin + 0.8°). Higher orders of the original (111) Bragg spot are now apparent. (F) The pattern from a thin ice assembly at a different azimuthal angle (φ + 45°), by rotating the substrate. From such an azimuthal search, the lack of other spots except for the original central one reflects the lack of a horizontal orientation order in the ice structure. (G) Rocking curve for a thin ice assembly obtained by collecting the central rod area of diffraction patterns as a function of θin. The equally spaced streaks are indicative of an ordered vertical stacking of ice. (H) Structures of cubic ice (hydrogen atoms are omitted) and graphite. See Results and Discussion for details.