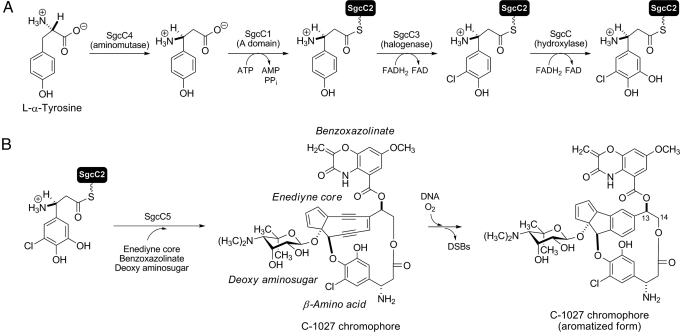
Fig. 1.
Biosynthesis of (S)-3-chloro-5-hydroxy-β-tyrosine and its incorporation into the C-1027 chromophore. (A) Biosynthesis of the β-amino acid moiety from l-α-tyrosine featuring 4 functionally assigned enzymes. (B) Attachment of (S)-3-chloro-5-hydroxy-β-tyrosine onto the enediyne core by SgcC5, leading to the C-1027 chromophore. The timing for each of the coupling steps is unknown. C-1027 undergoes an electronic rearrangement that generates a benzenoid biradical species that can abstract hydrogen atoms from DNA, leading to DNA breaks and an aromatized C-1027 chromophore. DSBs indicates double-strand breaks.