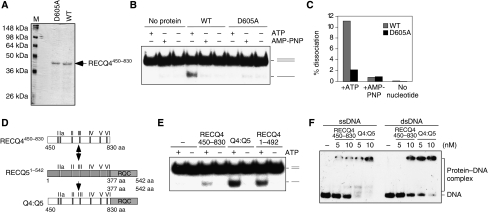
Figure 6.
DNA unwinding by the RECQ4 SFII domain. (A) Visualization of recombinant RECQ4450–830 fragment and the corresponding D605A mutant fragment purified from E. coli by SDS–PAGE and Commassie blue staining. (B) Helicase activities of the RECQ4 helicase domain fragment and D605A mutant (20 nM each) in the presence of ATP or AMP-PNP or without nucleotide cofactor. (C) Product formation in (C) was quantified by phosphorimaging. (D) Schematic diagram of the RECQ4 SFII helicase domain, RECQ51–542 fragment and the chimaera protein. The seven conserved helicase motifs were shown. (E) Helicase activities of the RECQ4450–830, RECQ4:RECQ5 chimaera and RECQ41–492 (20 nM each) in the presence or absence of ATP. (F) Binding of 32P-end-labelled single-stranded (left) and duplex oligos (right) by RECQ4450–830 fragment and RECQ4:RECQ5 chimaera protein. DNA–protein complex was analysed on 5% native PAGE.