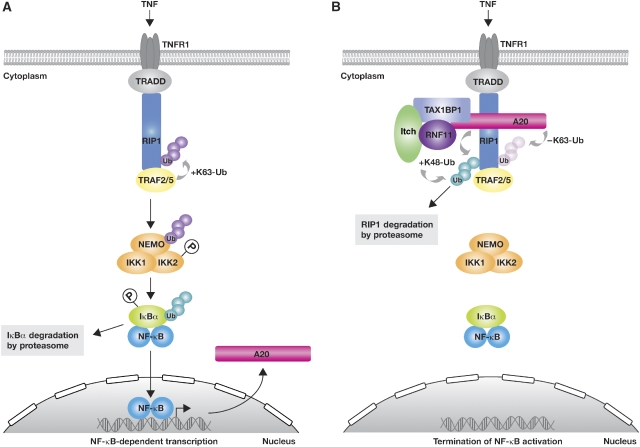
Figure 1.
Regulation of TNFR1 signalling by ubiquitination. (A) TNF stimulation induces binding of a TRADD–RIP1–TRAF2–TRAF5 complex to TNFR1. The TRAF proteins then catalyse the K63-linked polyubiquitination of RIP1, inducing RIP1 association with the IKK complex (IKK1/IKK2/NEMO), which is subsequently activated by a process that involves both its K63-linked ubiquitination and phosphorylation. IKK phosphorylates IκBα, triggering K48-linked polyubiquitination of IκBα, which induces its subsequent degradation by the proteasome. As a result, associated NF-κB dimers are freed to translocate into the nucleus and activate the transcription of NF-κB target genes, including A20. (B) The A20 ubiquitin-editing complex is critical to ensure that NF-κB activation induced by TNF is transient. This complex, comprising A20, TAX1BP1, Itch and RNF11, catalyses the removal of K63-linked ubiquitin chains from RIP1, utilizing the deubiquitinating activity of A20. Subsequently, A20 and Itch catalyse addition of K48-linked ubiquitin chains of RIP1, promoting its degradation by the proteasome and termination of NF-κB activation. K63-linked ubiquitin chains, purple; K48-linked ubiquitin chains, turquoise; P, phosphorylation; Ub, ubiquitin; +Ub, ubiquitination; −Ub, deubiquitination.