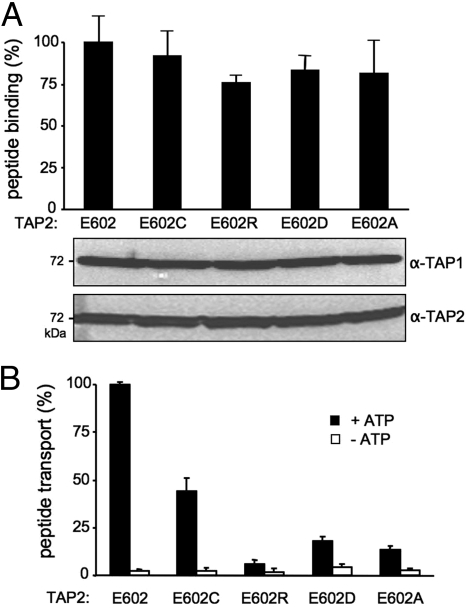
Fig. 2.
Function of the glutamate in the X-loop of TAP2 in the allosteric coupling between peptide binding and translocation. (A) Peptide binding of X-loop mutants of TAP2. TAP-containing membranes (35 μg of protein) were incubated with 1 μM radio-labeled RRYQKSTEL for 15 min at 4 °C. Unspecific binding was determined in the presence of an excess of RRYQKSTEL (200 μM). The lower portion shows the expression level of X-loop mutants analyzed by 10% SDS/PAGE (20 μg of protein per lane) followed by immunoblotting against TAP1 (mAb 148.3) and TAP2 (mAb 435.3). (B) Peptide transport of X-loop mutants of TAP2. TAP-containing membranes (150 μg of protein) were incubated with fluorescein-labeled peptide [1 μM RRYQNSTC(F)L] for 5 min at 32 °C in the presence (black bars) or absence (white bars) of 3 mM ATP. After lysis, N-core-glycosylated and thus translocated peptides were bound to ConA-beads and quantified by fluorescence detection after elution with methyl-α-d-mannopyranoside. Peptide binding and transport of TAP1(Cys-less)/TAP2(E602) was normalized to 100%. Data were collected from 3 independent experiments. The error bars represent the standard deviation.