Figure 9.
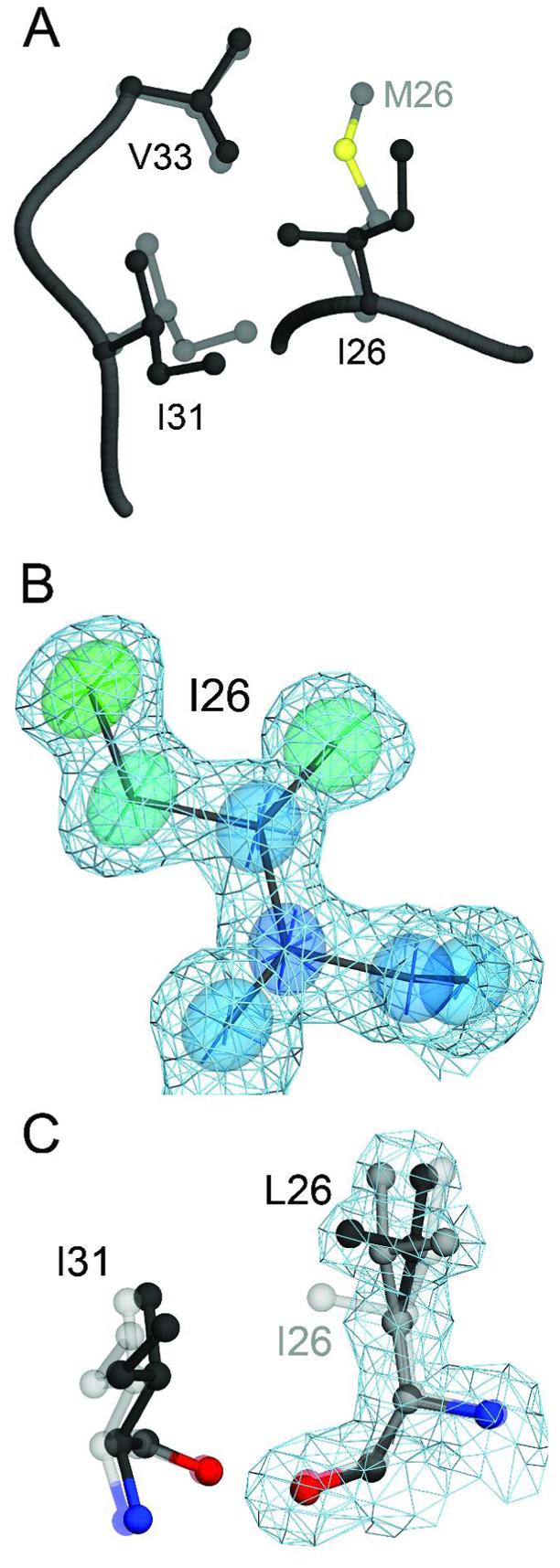
The M26I substitution results in packing defects in the core of DJ-1. Panel A shows a superposition of wild-type (1SOA(5); semi-transparent grey) and M26I DJ-1 (dark grey) in the region around the site of the substitution. The M26I substitution results in a ∼0.7 Å displacement of I31 to accommodate the Cγ2 atom of I26. In addition, the loss of the Sδ and Cε atoms of M26 creates a minor packing defect in the hydrophobic core of the protein. In Panel B, 2mFO-DFC electron density contoured at 1σ (blue) is shown with thermal ellipsoids calculated from the refined ADPs of I26, indicating that this residue is well-ordered in the crystal. The thermal ellipsoids are shown at the 60% probability level with their principal axes and are colored to indicate the magnitude of the total atomic displacement (blue; 6 Å2, red; 15 Å2). Panel C shows a superposition of M26I (semi-transparent grey) and the engineered M26L substitution, with alternate conformations for L26 shown in black and dark grey. The cavity created by the M26L substitution allows L26 to sample two rotameric conformations in the core of DJ-1, as shown by 2mFO-DFC electron density contoured at 1σ (blue) calculated from the 1.5 Å resolution crystal structure of M26L DJ-1. The figure was created with POVscript+((65).
