Abstract
Substantial production of new neurons in the adult mammalian brain is restricted to the olfactory system and the hippocampal formation. Its physiological and behavioural role is still debated. By comparing adult hippocampal neurogenesis (AHN) across many mammalian species, one might recognize a common function. AHN is most prominent in rodents, but shows considerable variability across species, being lowest or missing in primates and bats. The latter finding argues against a critical role of AHN in spatial learning and memory. The common functional denominator across all species investigated thus far is a strong decline of AHN from infancy to midlife. As predicted by Altman and colleagues in 1973, this implies a role in transforming juvenile unpredictable to predictable behaviour, typically characterizing mammalian behaviour once reproductive competence has been attained. However, as only a fraction of mammalian species has been investigated, further comparative studies are necessary in order to recognize whether AHN has a common unique function, or whether it mediates species-specific hippocampal functions.
Keywords: adult neurogenesis, evolution, life history, adult development, behaviour, flexibility
1. Introduction
Within the brain of adult mammals, proliferation of neurons occurs spontaneously in the walls of the lateral ventricle and the subgranular layer of the hippocampus. Cells from the ventricle walls migrate and integrate into the olfactory bulb, whereas new neurons in the subgranular layer differentiate into dentate gyrus granule cells of the hippocampus (figure 1). The functional significance of adult-born neurons in the hippocampus is still debated; several studies have found opposite results with respect to the role of newly generated cells in hippocampus-dependent learning and memory tasks (Saxe et al. 2006; Imayoshi et al. 2008). This raises the question of how important adult neurogenesis might be for animals living in their natural context, and to what extent experimentally obtained insights into the regulation and function of adult hippocampal neurogenesis (AHN) can help us to understand its relevance in the healthy and diseased human brain (Nottebohm 2002; Lindsey & Tropepe 2006). An obvious approach would be to compare wild species with differential demands for cognitive abilities and check for their levels of AHN, expecting those species excelling to exhibit extreme levels of AHN.
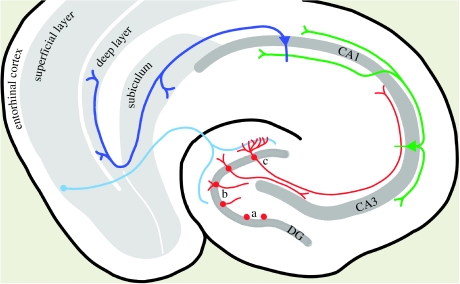
Figure 1.
Neurogenesis in the hippocampus. Progenitor cells divide in the subgranular layer of the hippocampus (a). Newborn neurons migrate a short distance into the granule cell (DG) layer (b) where they attain full mature state (c) by forming dendrites towards the molecular layer, receiving inputs from layer II of the entorhinal cortex (light blue), and connecting via mossy fibre pathway (red) to pyramidal cells of CA3. CA3 pyramidal cells send the information via Schaffer collaterals (green) to pyramidal cells of CA1, which in turn feedback the information to subiculum and deep layers of entorhinal cortex (dark blue). Neurogenesis occurs in the bottleneck of the unidirectional, trisynaptic loop of the hippocampus.
2. Functional correlates of adult hippocampal neurogenesis in wild mammalian species
The vast majority of studies of adult neurogenesis have been carried out in domesticated laboratory mice and rats. Both species show pronounced strain differences in their basal AHN, whose range of variation exceeds by far the known strain differences in behaviour (Kempermann et al. 1997; Perfilieva et al. 2001). AHN can be visualized immunohistochemically by endogenous proteins (figure 2a) or incorporated markers such as bromodeoxyuridine (BrdU). The direct comparisons of basal AHN rate among studies or species, however, are rare due to differences in markers, staining protocols, lack of standardization or missing unbiased counting procedures (for more details see Amrein et al. 2008). Still, AHN in wild rodents is well documented. Considerable species differences can be observed (figure 2a), and in some cases, different levels of AHN as predicted by their environment were found. High adult proliferation rates (up to 20 000 new cells daily) were found in wood mice patrolling large territories (figure 2a(i),(iv),b(i)) while lower levels were found in bank voles (figure 2a(ii),(v),b(ii)), and pine voles (Amrein et al. 2004). Studies in two squirrel species, with different territory sizes and caching behaviour, showed that chipmunks with small territories with a single food cache had lower basal proliferation rate than squirrels using multiple storage places located in larger territories (Barker et al. 2005). Seasonally changing requirements for spatial memory did not lead to altered neurogenesis in wild squirrels (Lavenex et al. 2000). Most bats lack neurogenesis in the hippocampus (figure 2a(iii),(vi),b(iii), and Amrein et al. 2007), despite showing precise spatial memory for food sources in the wild and laboratory (Thiele & Winter 2005). Possibly, spatial, episodic and contextual memory may be more dependent on neurogenesis than spatial navigation (Wojtowicz et al. 2008). This might be particularly important for predated rodents that must constantly relate danger to changing locations and stimuli, thus creating a particularly high demand for AHN. However, the attempt of linking extreme rates of AHN with any type of hippocampus-dependent cognitive abilities faces one big problem: primates, and in particular humans, show comparatively low levels of AHN (Eriksson et al. 1998; Gould et al. 1999), which, in humans, seemingly becomes rudimentary after 30 years of age (Fahrner et al. 2007).
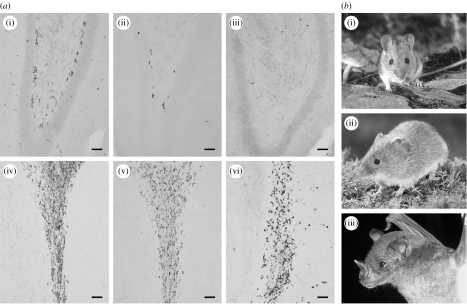
Figure 2.
(a) Extremes in hippocampal proliferation activity as revealed by Ki-67 immunohistochemistry. Adult yellow-necked wood mice (Apodemus flavicollis; (i),(iv)) show extreme proliferation activity in the dentate gyrus (i) and the rostral migratory stream, RMS (iv). In adult bank voles (Myodes glareolus, formerly Clethrionomys glareolus; (ii),(v)), proliferation activity in the dentate gyrus is lower (ii), but equal to wood mice in the RMS (v). In the hippocampus of a pale spear-nosed bat (Phyllostomus discolour; (iii),(vi)), a few Ki-67 positive cells can be seen in the hilus, but not in the subgranular layer of the dentate gyrus (iii). Proliferation activity in the RMS (vi) is similar to that in the mice. Sagittal sections, scale bar=50 μm. (b) Similar sized mammals with extremes in AHN and lifespan. (i) Short-lived yellow-necked wood mice and (ii) bank voles show high and low level of AHN. (iii) Long-lived pale spear-nosed bats do not have proliferation activity in the hippocampus. Photo (i) and (ii): Rollin Verlinde; photo (iii): Katja Rex.
3. Is age dependency of mammalian adult neurogenesis associated with behavioural changes in life history?
Thus far, an age-dependent decline of AHN is the only common finding in all species investigated. Protracted neurogenesis of granule cells peaks at puberty and declines dramatically thereafter, as documented in mice, rats and monkeys (Kuhn et al. 1996; Gould et al. 1999; Ben Abdallah et al. in press), albeit with considerable species differences. Wild old wood mice, voles, chipmunks and squirrels show a decline in ongoing proliferation compared with that of young and adults (Amrein et al. 2004; Barker et al. 2005). In shrews, neurogenesis ceases completely once the animals have overwintered (Bartkowska et al. 2008). A decline in ongoing proliferation appears to be a truly general phenomenon in laboratory bred as well as wild animals and humans (Seress et al. 2001; Fahrner et al. 2007). In order to understand the functional relevance of AHN across species, age, lifespan and mortality rate of the animals must be taken into account. Age dictates basal cell proliferation rates, average lifespan might be correlated with the number of precursor cells, and mortality rate might indicate how essential young neurons in the hippocampus are for the survival of the animals.
Therefore, one would expect AHN in many mammalian species to be associated with the change of behavioural traits that characterize the transition from juvenile to adult behaviour. The idea is not new: Altman and colleagues noted that the function of hippocampal maturation might be ‘transforming reckless juveniles into cautious adults’ (Altman et al. 1973). Later on, Lipp & Wolfer (1995) tried to fit adult changes in hippocampal circuitry to evolutionary mechanisms. They hypothesized that high behavioural flexibility during late adolescence and young adulthood improves the chance of establishing ecological and social niches facilitating reproduction. After having found such niches, however, radical changes of the behavioural profile are not likely to enhance reproductive success. Thus, brain processes that strengthen acquired habits probably increase the thresholds for behavioural change. The age-dependent decline in AHN might reflect a late form of selective developmental stabilization (Changeux & Danchin 1976). Networks containing newly formed dentate granule cells might form a mosaic of differentially activated channels along the many parallel intrahippocampal (trisynaptic) loops (figure 1). Continued neuronal activity along these channels might then result in local downregulation of granule cell proliferation, resulting in predictable and stable topographical patterns of hippocampal activity. Ultimately, this leads to increased predictability of activities characterizing mature adult behaviour. Late developmental disappearance of AHN would thus correspond to a slow form of neuronal plasticity—a morphological learning, possibly accompanied by other age-dependent changes in the hippocampal mossy fibre system (Wolfer & Lipp 1995) and other brain structures controlling the behaviour such as the prefrontal cortex.
This hypothesis might explain a considerable portion of the differential dynamics of AHN in mammals. Most rodents are short-lived species with a reproductive peak lasting one season only. This leaves a period of few months during which locally appropriate behavioural habits can be developed. These behavioural habits must relate to available food sources, predator pressure and social interactions with conspecifics. Once acquired, they may be useful for another year at best. On the other hand, bats have a long life, but acquire sexual maturity in a few months, even if they normally do not reproduce before 1 year old. Given the adaptation to very specialized ecological niches and relative lack of predator pressure, behavioural routines acquired during a few months may be sufficient for the rest of life, not needing further modifications. Finally, primates with their (genetically determined) protracted period of development may have a longer window of AHN and routine forming, but reaching sexual maturity and full reproductive sexual status will reduce eventually this process to very low levels.
Clearly, such hypotheses require comparative verification. An obvious step is to investigate long- and short-living rodent species sharing a similar environment. Another one would be to compare predators versus predated species, including small variants such as weasels versus mice, or large animals such as lions versus antelopes. Finally, the search may be extended to include species with socially complex versus solitary lifestyles. Whatever the outcome will be, it offers the promise to eliminate—as shown for bats—hypotheses about a general physiological role of AHN in mammals. Hopefully, a general rule of function will emerge.
Acknowledgments
I.A and H.P.L. were supported by the Swiss National Science Foundation and the NCCR ‘Neural Plasticity and Repair’. We thank Fabienne Klaus and Thomas Hauser for their critical reading, Heinz Sonderegger for his drawing. Animal photos were kindly provided by Rollin Verlinde (vildaphoto.net) and Katja Rex.
Footnotes
One contribution of 10 to a Special Feature on ‘Brain evolution’.
References
- Altman J., Brunner R.L., Bayer S.A. The hippocampus and behavioral maturation. Behav. Biol. 1973;8:557–596. doi: 10.1016/s0091-6773(73)80144-0. doi:10.1016/S0091-6773(73)80144-0 [DOI] [PubMed] [Google Scholar]
- Amrein I., Slomianka L., Poletaeva I.I., Bologova N.V., Lipp H.P. Marked species and age-dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-living rodents. Hippocampus. 2004;14:1000–1010. doi: 10.1002/hipo.20018. doi:10.1002/hipo.20018 [DOI] [PubMed] [Google Scholar]
- Amrein I., Dechmann D.K., Winter Y., Lipp H.P. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera) PLoS ONE. 2007;2:e455. doi: 10.1371/journal.pone.0000455. doi:10.1371/journal.pone.0000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I., Boonstra R., Lipp H.P., Wojtowicz J.M. Adult hippocampal neurogenesis in natural populations of mammals. In: Gage F.H., Kempermann G., Song H., editors. Adult neurogenesis. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2008. pp. 645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J.M., Wojtowicz J.M., Boonstra R. Where's my dinner? Adult neurogenesis in free-living food-storing rodents. Genes Brain Behav. 2005;4:89–98. doi: 10.1111/j.1601-183X.2004.00097.x. doi:10.1111/j.1601-183X.2004.00097.x [DOI] [PubMed] [Google Scholar]
- Bartkowska K., Djavadian R.L., Taylor J.R.E., Turlejski K. Generation recruitment and death of brain cells throughout the life cycle of Sorex shrews (Lipotyphla) Eur. J. Neurosci. 2008;27:1710–1721. doi: 10.1111/j.1460-9568.2008.06133.x. doi:10.1111/j.1460-9568.2008.06133.x [DOI] [PubMed] [Google Scholar]
- Ben Abdallah, N. M.-B., Slomianka, L., Vyssotski, A. L. & Lipp, H.-P. In press. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging (doi:10.1016/j.neurobiolaging.2008.03.002) [DOI] [PubMed]
- Changeux J.-P., Danchin A. Selective stabilization of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. doi:10.1038/264705a0 [DOI] [PubMed] [Google Scholar]
- Eriksson P.S., Perfilieva E., Bjork-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. doi:10.1038/3305 [DOI] [PubMed] [Google Scholar]
- Fahrner A., Kann G., Flubacher A., Heinrich C., Freiman T.M., Zentner J., Frotscher M., Haas C.A. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp. Neurol. 2007;203:320–332. doi: 10.1016/j.expneurol.2006.08.023. doi:10.1016/j.expneurol.2006.08.023 [DOI] [PubMed] [Google Scholar]
- Gould E., Reeves A.J., Fallah M., Tanapat P., Gross C.G., Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc. Natl Acad. Sci. USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. doi:10.1073/pnas.96.9.5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I., et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. doi:10.1038/nn.2185 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H.G., Gage F.H. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc. Natl Acad. Sci. USA. 1997;94:10 409–10 414. doi: 10.1073/pnas.94.19.10409. doi:10.1073/pnas.94.19.10409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H.G., Dickinson-Anson H., Gage F.H. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P., Steele M.A., Jacobs L.F. Sex differences, but no seasonal variations in the hippocampus of food- caching squirrels: a stereological study. J. Comp. Neurol. 2000;425:152–166. doi:10.1002/1096-9861(20000911)425:1<152::AID-CNE13>3.0.CO;2-Y [PubMed] [Google Scholar]
- Lindsey B.W., Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog. Neurobiol. 2006;80:281–307. doi: 10.1016/j.pneurobio.2006.11.007. doi:10.1016/j.pneurobio.2006.11.007 [DOI] [PubMed] [Google Scholar]
- Lipp H.-P., Wolfer D.P. New paths towards old dreams: microphrenology or the study of intact brains in intact worlds. In: Alleva E., Fasolo A., Lipp H.-P., Nadel L., Ricceri L., editors. Behavioural brain research in naturalistic and semi-naturalistic settings: possibilities and perspectives. Kluwer; Dordrecht, The Netherlands: 1995. pp. 1–39. [Google Scholar]
- Nottebohm F. Why are some neurons replaced in adult brain? J. Neurosci. 2002;22:624–628. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfilieva E., Risedal A., Nyberg J., Johansson B.B., Eriksson P.S. Gender and strain influence on neurogenesis in dentate gyrus of young rats. J. Cereb. Blood Flow Metab. 2001;21:211–217. doi: 10.1097/00004647-200103000-00004. doi:10.1097/00004647-200103000-00004 [DOI] [PubMed] [Google Scholar]
- Saxe M.D., et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl Acad. Sci. USA. 2006;103:17 501–17 506. doi: 10.1073/pnas.0607207103. doi:10.1073/pnas.0607207103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L., Abraham H., Tornoczky T., Kosztolanyi G. Cell formation in the human hippocampal formation from mid-gestation to the late postnatal period. Neuroscience. 2001;105:831–843. doi: 10.1016/s0306-4522(01)00156-7. doi:10.1016/S0306-4522(01)00156-7 [DOI] [PubMed] [Google Scholar]
- Thiele J., Winter Y. Hierarchical strategy for relocating food targets in flower bats: spatial memory versus cue-directed search. Anim. Behav. 2005;69:315–327. doi:10.1016/j.anbehav.2004.05.012 [Google Scholar]
- Wojtowicz J.M., Askew M.L., Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur. J. Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. doi:10.1111/j.1460-9568.2008.06128.x [DOI] [PubMed] [Google Scholar]
- Wolfer D.P., Lipp H.-P. Evidence for physiological sprouting of hippocampal mossy fiber collaterals in the guinea pig during puberty and adulthood. Hippocampus. 1995;5:329–340. doi: 10.1002/hipo.450050406. doi:10.1002/hipo.450050406 [DOI] [PubMed] [Google Scholar]