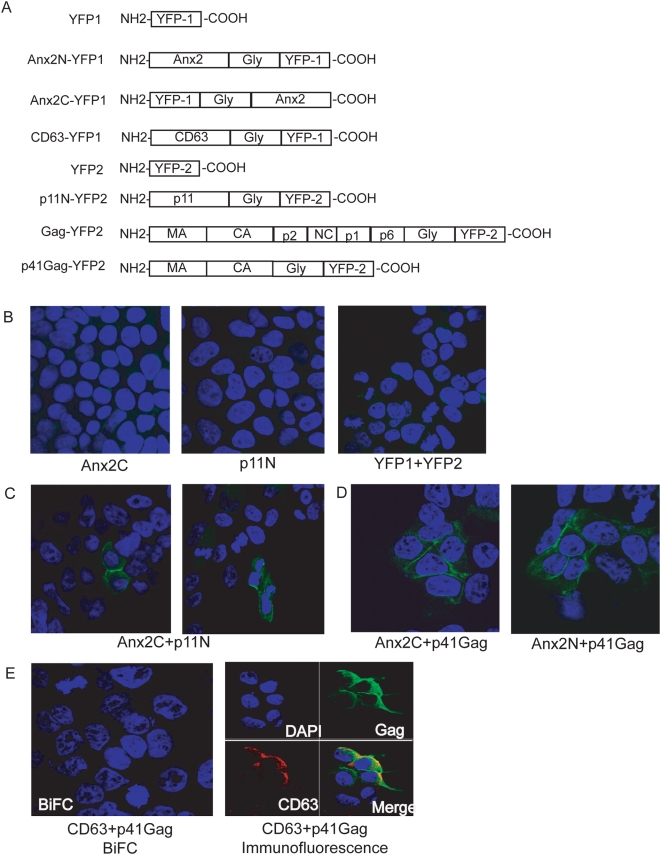
Figure 2. Bimolecular fluorescence complementation (BiFC) showed that Anx2 and p41Gag interacted at the plasma membrane of 293T cells.
293T cells transfected with the indicated constructs were stained with DAPI and visualized by confocal microscopy 36 hours post-transfection. (A) BiFC constructs. N and C in the designation denote location at the N- or C-terminus of the construct, respectively. Gly: Glycine linker (not to scale). (B) No YFP fluorescence was detected in 293T cells transfected with single constructs or in cells cotransfected with both YFP fragments. (C) YFP fluorescence, indicating an interaction between p11 and Anx2, was detected in 293T cells cotransfected with p11 and Anx2 BiFC constructs (positive control). (D) YFP fluorescence, indicating an interaction between p41Gag and Anx2, was detected at the plasma membrane of 293T cells cotransfected with p41Gag and Anx2 BiFC constructs. (E) No YFP fluorescence was detected in cells cotransfected with p41Gag and CD63 BiFC constructs (left panel), despite colocalized expression of CD63 and p41Gag as demonstrated by immunolabeling (right panel). These data indicated CD63 and p41Gag did not interact and confirmed the specificity of the fluorescence complementation between p41Gag and Anx2.