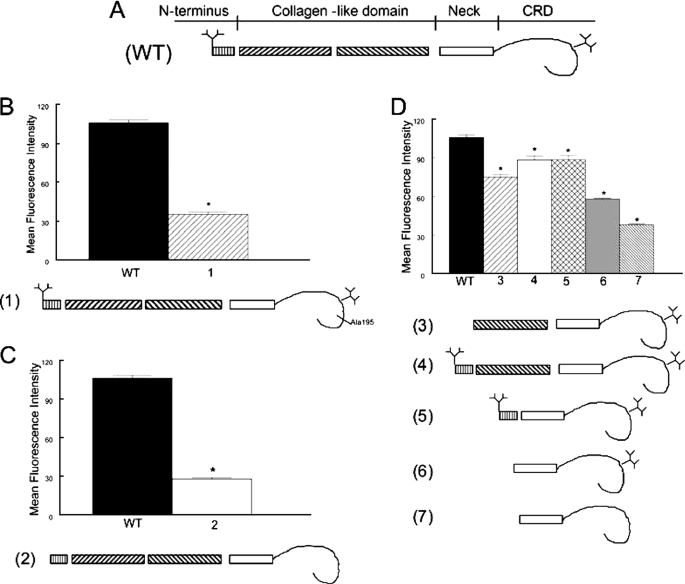
FIGURE 6.
Multiple domains of SP-A contribute to binding to CR3. The interaction of fluorescein-labeled SP-A with CR3 was determined by flow cytometry. The structure of the monomeric subunit of wild-type (WT) recombinant SP-A is shown in panel A. Note the branching N-linked carbohydrates attached to the N terminus and the CRD of the protein. In panel B, an E195A mutation that incapacitates the lectin function (1) of the CRD greatly reduced binding of SP-A to CR3. In panel C, tandem N1T and N187S mutations of SP-A, which prevent attachment of N-linked carbohydrates (2) also markedly reduced binding of SP-A to CR3. In panel D, truncated SP-A proteins lacking the N-terminal domain, the N-terminal N-linked carbohydrate attachment site, and first half of the collagen domain but possessing an intact Asn187 carbohydrate attachment (3), lacking the first half of the collagen domain but possessing the N-terminal segment, and both N-linked carbohydrate moieties (4), or lacking the collagen domain but possessing the N-terminal segment, and both carbohydrate moieties (5) retained significant CR3 binding activity. Mutations that resulted in a deletion of the N-terminal segment, the collagen-like domain, and the N-terminal (N1T) carbohydrate moiety (6) reduced binding of SP-A to CR3, and the binding activity that was further diminished by introduction of an additional N187S mutation prevented attachment of the CRD (7). These data implicate the N-linked carbohydrates, the CRD, and perhaps the neck domain in the interaction of SP-A with CR3. Data are mean ± S.E. with n = 4 experiments per group, *, p < 0.05 compared with binding of rat recombinant SP-A.