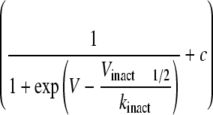
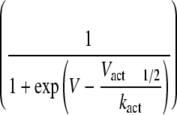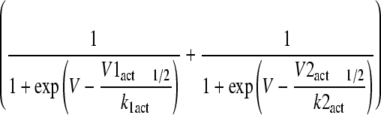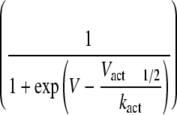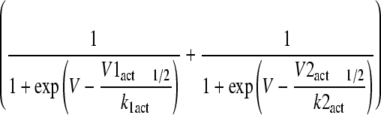Representative plots of activation and steady-state inactivation curves
for Nav1.4 and Nav1.8 channels. A, pulse
protocols for activation curves (
top) and inactivation curves
(
bottom).
B and
C, symbols indicate data in the
absence (
solid lines and
closed squares) and presence
(
dotted lines and
open circles) of 0.1 μ
m
CTX3C. Boltzmann functions (
smooth lines) were fitted to each set of
activation curves
or inactivation curves
In the case of ciguatoxin-modified Na
v1.8 channels, activation
curves were fitted by a sum of two Boltzmann distributions
During standard double-pulse protocols for the steady-state inactivation
measurement, giving conditioning pulses of –120 to –100 mV,
increased the availability of Na
+ channels in the presence of CTX3C
before the transition to inactivation, especially in Na
v1.8, making
a peak in the curve at around –100 mV. In this case, a fit was obtained
by not fitting data points showing potentials more negative than –100
mV. The parameters obtained by the fitting procedure for all of the constructs
are shown in
Table 1 for the
activation curve and in
Table 2
for the steady-state inactivation curve. The activation curves were calculated
from the peak
INa value obtained at each membrane
potential divided by the driving force. The steady-state inactivation curves
were obtained by a double-pulse protocol. The
INa evoked
with a test pulse to –20 mV was preconditioned by a 500-ms clamp step to
a variable membrane potential (–160 to –20 mV in 20-mV increments)
with a gap of 0.25 ms, and normalized by the unconditioned current elicited
with a test pulse from the holding potential (–100 mV).