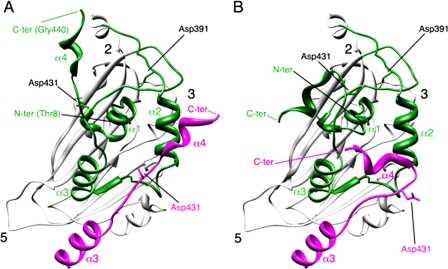
FIGURE 1.
Conformational flexibility of the VP2 C-terminal α-helix around its cleavage site. A, bottom view, facing the inner surface of the viral capsid, of the VP2 protein x-ray model (PDB entry 2GSY). α-Helices (1-4) of domain B (green) are indicated for a VP2 chain; the candidate catalytic residues Asp-391 and Asp-431 are also indicated. α3 and α4 helices of the closer neighboring VP2 chain (magenta) that projects toward the 3-fold axis of the trimer are shown, and to simplify the view, only its Asp-391 residue is indicated. The locations of 5-, 3-, and 2-fold axes of icosahedral symmetry are indicated. B, same as panel A, but the C-terminal α4 helices of VP2 chains were remodeled after fitting the x-ray model into an equivalent cryo-electron microscopy map (21).