Abstract
Nuclear retinoic acid receptor α (RARα) activates gene expression through dynamic interactions with coregulatory protein complexes, the assembly of which is directed by the ligand and the AF-2 domain of RARα. Then RARα and its coactivator SRC-3 are degraded by the proteasome. Recently it has emerged that the proteasome also plays a key role in RARα-mediated transcription. Here we show that SUG-1, one of the six ATPases of the 19 S regulatory complex of the 26 S proteasome, interacts with SRC-3, is recruited at the promoters of retinoic acid (RA) target genes, and thereby participates to their transcription. In addition, SUG-1 also mediates the proteasomal degradation of SRC-3. However, when present in excess amounts, SUG-1 blocks the activation of RARα target genes and the degradation of RARα that occurs in response to RA, via its ability to interfere with the recruitment of SRC-3 and other coregulators at the AF-2 domain of RARα. We propose a model in which the ratio between SUG-1 and SRC-3 is crucial for the control of RARα functioning. This study provides new insights into how SUG-1 has a unique role in linking the transcription and degradation processes via its ability to interact with SRC-3.
Retinoic acid (RA)6 influences the proliferation, differentiation, and apoptosis of a variety of cell types through modifications in the expression of subsets of target genes. The RA response is mediated by two classes of nuclear receptors, RARs (α, β, and γ) and RXRs (α, β, and γ), which function as ligand-dependent heterodimeric RAR/RXR transcription activators (1, 2). The basic mechanism of RARs transcriptional activity relies on their ability to bind to cognate DNA response elements located in the promoters of target genes and to undergo major conformational changes subsequently to ligand binding (3). The most striking change observed upon ligand binding is the swing of helix 12 named AF-2 located at the C-terminal end of the ligand binding domain, which generates a new interaction surface for coactivators, which include the steroid receptor coactivator (SRC)/p160 family (SRC-1/NCoA1, SRC-2/TIF2/GRIP-1, and SRC-3/p/CIP/RAC3/ACTR/AIB-1/TRAM-1) and p300/CBP (4, 5). These coactivators then recruit a battery of intermediary proteins, including chromatin remodelers and modifiers that act in a coordinated and combinatorial manner to decompact chromatin and direct RNA polymerase II and the general transcription factors to the promoter (6-8).
Emerging evidence indicates that the proteasome system plays an important role in transcription of nuclear receptor target genes (9-12). The 26 S proteasome is composed of a 20 S proteolytic core and a 19 S regulatory complex that consists of a lid and a base of six subunits with ATPase activity. These ATPases recognize the ubiquitinated proteins and direct them to the 20 S core for subsequent degradation (reviewed in Ref. 13). However, mounting evidence indicates that the proteasome also plays a role in transcription through mechanisms that do not involve proteolysis (14-16). The 26 S proteasome is involved in the degradation of RARs (17, 18), corepressors (19), and coactivators such as SRC-3 (20) that occurs in response to RA, but its relevance in RAR-mediated transcription is not yet clear (11, 21). Here we identified a new mechanism underlying the regulation of RARα target genes, in which SUG-1, one of the six AAA ATPases of the 19 S regulatory complex of the 26 S proteasome, links the transcription and degradation processes via its ability to interact with the p160 coactivator SRC-3.
EXPERIMENTAL PROCEDURES
Plasmids and Reagents—The pSG5-based expression vectors for mouse (m) RARα1, mRARαΔH12, mSUG-1, and human (h) SRC-1 were previously described (18, 20, 22) as well as the vector encoding B10-HA-FLAG-tagged SRC-3 and the DR5-tk--Luc reporter gene (20). The prokaryotic vector encoding SUG-1 fused to GST in the pGEX-2T plasmid was described before (22). All trans-RA was from Sigma-Aldrich.
Antibodies—Monoclonal antibodies against the AB region of RARα (MAb10α (AB)), SUG-1, and polyclonal antibodies against the F region of RARα (RPα (F)) were previously described (18, 20, 22). Mouse monoclonal antibodies against SRC-3 and SRC-2 were from BD Transduction Laboratories. Those against SRC-1 were from Affinity Bioreagents. Anti-FLAG antibodies were from Sigma. Antibodies against β-actin (C-11) were from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal antibodies against RARα (C-20) and SRC-3 used in the ChIP experiments were from Santa Cruz Biotechnology, whereas those against SUG-1 were from Biomol Research Laboratories.
Cells, Transfections, Immunoprecipitations, and Immunoblotting—MCF7, HeLa, and COS-1 cells were cultured under standard conditions and transiently transfected using the DMRIE-C reagent, according to the manufacturer's protocol (Invitrogen). Luciferase activity was determined according to standard procedures. Whole cell and nuclear extracts were prepared and subjected to immunoprecipitation and immunoblotting as in a previous study (23).
GST Pulldown Assays—Equimolar GST or GST-SUG-1 fusion proteins produced in Escherichia coli were purified on glutathione-Sepharose 4B beads (Amersham Biosciences) and incubated with extracts from COS-1 cells overexpressing SRC-3 as described (23). Bound proteins were analyzed by immunoblotting.
siRNA—The SMARTpool small interfering RNA (siRNA) against human NCOA3/SRC-3 (M-003759-02), NCOA2/SRC-2 (M-020159-01), NCOA1/SRC-1 (M-005196-03), and SUG-1 (M-009484-02) were purchased from Dharmacon (Lafayette, CO) as well as the control non-targeting siRNA pool (D-001206-13). siRNAs (50 nm) were transfected into MCF7 and HeLa cells according to the manufacturer's protocol.
RNA Isolation and Quantitative RT-PCR—Total RNAs were isolated and subjected to real-time qRT-PCR as previously described (23). Primer sequences were as follows: human CYP26A1, 5′-GAAAGTGCGAGAAGAGCTGAA-3′ and 5′-CCTTGGGAATCTGGTATCCAT-3′; human RARα2, 5′-TTGGAATGGCTCAAACCAC-3′ and 5′-TTGTAGATGCGGGGTAGAGG-3′.
ChIP—Subconfluent MCF7 cells were treated with RA (10-7m) and ChIP experiments were next performed according to the protocol described by Upstate. In Re-ChIP experiments, complexes were eluted by incubation for 30 min at 37 °C in 250 ml of 95 mm NaHCO3 containing 1% SDS, diluted 20 times with Re-ChIP buffer (1% Triton X-100, 50 mm Tris-HCl (pH 7.5), 2 mm EDTA, 150 mm NaCl) and subjected again to the ChIP procedure. The primer pairs used for qPCR amplification were as follows: Cyp26A1 R1, 5′-GCGGAACAAACGGTTAAAGA-3′ and 5′-GCAGTACAGGTCCCAGAGCTT-3′; Cyp26A1 R2, 5′-GAGTTCACTCGGATGTCACG-3′ and 5′-CTGGGCAGGGTTTCAGTCT-3′; RARβ2, 5′-CGATCCCAAGTTCTCCCTTC-3′ and 5′-CAGACTGGTTGGGTCATTTG-3′. Occupancy of the promoters was calculated by normalizing the PCR signals from the immunoprecipitation samples to the signals obtained from the input DNA. The specificity of the experimental conditions was checked in the absence of antibodies and with the promoter of the control 36B4 gene, which does not contain any RA response element (RARE). For ChIP-Western experiments, the precipitated chromatin complexes were proceeded as in a previous study (24), and bound proteins were revealed by immunoblotting.
RESULTS
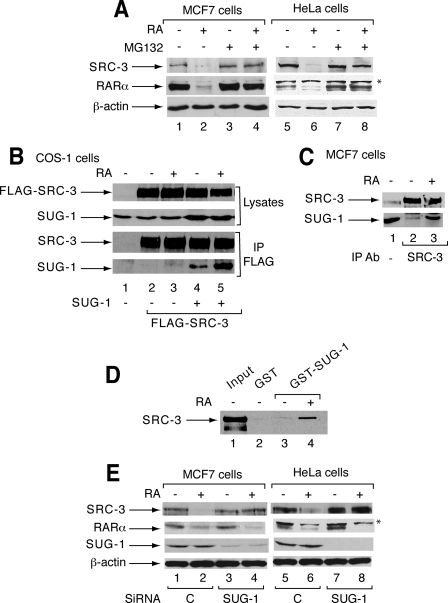
SUG-1 Interacts with SRC-3 and Contributes to the RA-induced Proteasomal Degradation of SRC-3—SRC-3 is degraded in several cell lines after 10-16 h of RA treatment (Fig. 1A) (20). It is prevented by the proteasome inhibitor MG132 (Fig. 1A), and thus occurs through the proteasome. Given the ability of SUG-1, one of the six AAA ATPases of the 19 S regulatory complex of the 26 S proteasome, to interact with several transcription factors (22, 25, 26), we investigated whether this subunit is involved in the degradation of SRC-3.
FIGURE 1.
SUG-1 interacts with SRC-3 and contributes to RA-induced degradation of SRC-3. A, in MCF7 and HeLa cells, MG132 reverses the RA-induced degradation of SRC-3 and RARα (16 h). B, COS-1 cells were transfected with the FLAG-SRC-3 vector in the absence or presence of SUG-1 and treated or not with RA (1 h). Nuclear extracts were immunoprecipitated with FLAG antibodies and analyzed by immunoblotting. The two upper panels correspond to aliquots (10%) of unprecipitated extracts. C, in MCF7 cells, SUG-1 interacts with SRC-3 in coimmunoprecipitation experiments performed with SRC-3 antibodies. D, immobilized GST and GST-SUG-1 proteins were incubated with extracts from COS-1 cells overexpressing SRC-3. Bound SRC-3 was analyzed by immunoblotting. Lane 1 corresponds to 5% of the loaded material. E, MCF7 and HeLa cells were transfected with control or SUG-1 SMARTpool siRNA (50 nm) and RA-treated for 16 h. Knockdown of SUG-1 was analyzed by immunoblotting as well as the expression and degradation of SRC-3 and RARα. *, a nonspecific band recognized by the RARα antibodies.
First we examined the ability of SUG-1 to interact with SRC-3 in coimmunoprecipitation experiments performed with extracts from COS-1 cells overexpressing SUG-1 and FLAG-SRC-3. SUG-1 was retained by SRC-3 in the absence of ligand, and addition of RA further stimulated this interaction (Fig. 1B). Similar results were obtained with endogenous SRC-3 and SUG-1 proteins in MCF7 cells (Fig. 1C). That SUG-1 interacts with SRC-3 was corroborated in in vitro pulldown experiments using the GST-SUG-1 fusion protein (Fig. 1D). No interaction could be detected with the other coactivator SRC-1 (data not shown), which is not degraded in response to RA (20).
Then we investigated whether SUG-1 is involved in the RA-induced degradation of SRC-3 by using RNA interference. Selective knockdown of SUG-1 in MCF7 or HeLa cells with SMARTpool siRNAs did not affect the basal levels of SRC-3 but inhibited the RA-induced degradation of the coactivator (Fig. 1E). Note that the RA-induced degradation of RARα, which also involves the proteasome (18) (Fig. 1B), was not abrogated but rather enhanced upon knockdown of SUG-1 (Fig. 1E) suggesting an unexpected degree of target specificity.
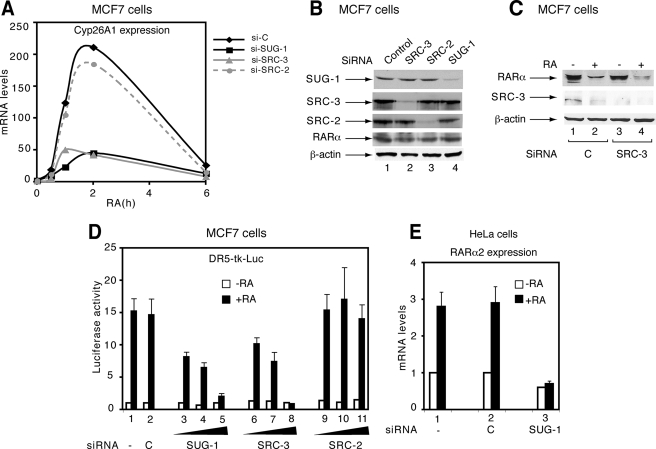
SUG-1 and SRC-3 Contribute to the Transcription of RA Target Genes—Given that SRC-3 is a coactivator for RARα and that SUG-1 functions in a number of transcription programs (25, 26), we investigated whether SUG-1 also contributes to the transcription of RA target genes. In MCF7 cells, RA treatment induces rapidly (within 1 h) the expression of the Cyp26A1 gene that is the paradigm of the RA target genes, as assessed by qRT-PCR (Fig. 2A). Selective knockdown of SUG-1 (Fig. 2B) led to a marked reduction of the RA-induced expression of Cyp26A1 (Fig. 2A). Knockdown of SRC-3, which does not affect basal RARα levels (Fig. 2B) or RA-induced degradation of RARα (Fig. 2C), also reduced efficiently Cyp26A1 expression (Fig. 2A). This suggests that SUG-1 would contribute to RARα-dependent transcriptional activation as efficiency as SRC-3 (20, 27). In contrast, knockdown of the other p160 coactivators, SRC-2 and SRC-1, had no effect (Fig. 2, A and B), in line with the concept that SRC proteins, though sharing a high degree of similarity, exert different functions in nuclear receptor-regulated gene transcription (28). That both SUG-1 and SRC-3 influence RARα-mediated transcription has been confirmed in MCF7 cells cotransfected with a Luciferase reporter gene driven by a DR5 RA response element (Fig. 2D) and in HeLa cells with other endogenous RARα target genes (the RARα2 gene) (Fig. 2E).
FIGURE 2.
Knockdown of SUG-1 inhibits the activation of RA target genes. A, the RA-induced expression of the endogenous Cyp26A1 gene was measured by qRT-PCR in MCF7 cells transfected with control, SUG-1, SRC-3, or SRC-2 SMARTpool siRNAs. The results are an average of two experiments that agreed within 15%. B, the efficiency and specificity of the knockdown was checked by immunoblotting. C, in MCF7 cells, knockdown of SRC-3 with SMARTpool siRNAs does not affect the RA-induced degradation of RARα. D, MCF7 cell knockdown for SUG-1, SRC-3, or SRC-2, was cotransfected with a DR5-tk-Luc reporter gene, treated with RA, and analyzed for Luciferase activity. The values are the mean ± S.D. of triplicate experiments. E, in HeLa cells, knockdown of SUG-1 with SMARTpool siRNA blocks the RA-induced expression of the endogenous RARα2 gene, measured by qRT-PCR. The values are the mean ± S.D. of triplicate experiments.
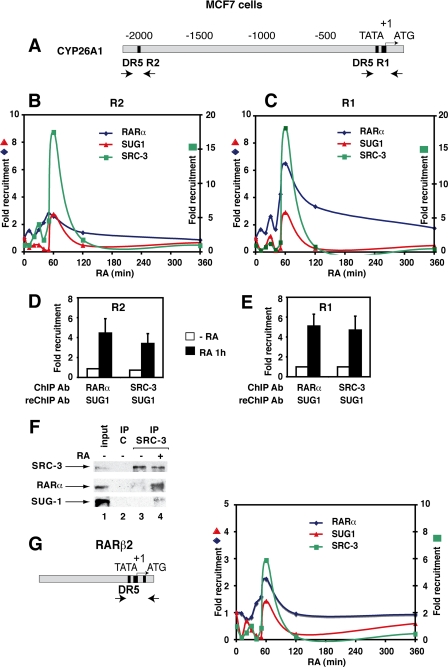
SUG-1 Is Recruited with SRC-3 to the Promoters of RA Target Genes—Next, we used chromatin immunoprecipitation (ChIP) experiments to investigate further the role played by SUG-1 in the transcription of endogenous RA target genes. The Cyp26A1 gene promoter contains two functional DR5 RAREs (Fig. 3A), a proximal one (R1) and a more distal one (R2), that work synergistically to provide a maximal response to RA in vivo (29). We assessed the SUG-1 and SRC-3 occupancy of these promoter regions in MCF7 cells treated with RA during a time course. After RA addition, there was an enrichment of SUG-1 bound at both the R1 and R2 regions, which peaked at 60 min (Fig. 3, B and C). The levels of R1 and R2 bound to SRC-3 also increased with a peak that was concomitant with that of SUG-1. To investigate whether SUG-1 is present in the same complex with SRC-3 on the promoter, MCF7 cells were subjected to sequential ChIP (Re-ChIP) experiments first with SRC-3 and then with SUG-1 antibodies (Fig. 3, D and E). At 60 min following RA addition, both the R1 and R2 regions were specifically enriched, indicating that SUG-1 and SRC-3 form a complex on DNA in an RA-dependent manner.
FIGURE 3.
In vivo, SUG-1 is co-recruited with SRC-3 to the promoter of RARα target genes. A, schematic representation of the promoter regions of the Cyp26A1 gene with the primer pairs used for their amplification. B and C, kinetic ChIP experiments performed with RA-treated MCF7 cells and determining the recruitment of RARα, SRC-3, and SUG-1 to the R1 and R2 regions. Values are expressed as -fold enrichment relative to untreated cells and correspond to a representative experiment among 3. D and E, Re-ChIP experiments performed with the indicated antibodies, showing that, at 60 min following RA addition, DNA-bound SRC-3 and RARα are associated with SUG-1. Values are expressed as -fold enrichment relative to control Re-ChIP experiments and are the mean ± S.D. of triplicate experiments. F, ChIP-Western experiments performed with MCF7 cells treated or not with RA for 60 min. The complexes immunoprecipitated with SRC-3 antibodies were analyzed by immunoblotting as indicated. Lane 1 corresponds to aliquots (10%) of unprecipitated extracts. G, kinetic ChIP experiments determining the recruitment of RARα, SRC-3, and SUG-1 to the RARβ2 promoter as in A. The promoter with the DR5 RARE is also shown.
RARα was also recruited in the same time slot (Fig. 3, B and C), in line with our previous results (30). In Re-ChIP experiments performed first with RARα antibodies and then with SUG-1 antibodies, enrichment was also observed at 60 min (Fig. 3, D and E). Collectively, these results suggest that SUG-1 is recruited within complexes containing both SRC-3 and RARα. This has been corroborated in ChIP Western experiments (Fig. 3F): immunoprecipitated SRC-3 retained both RARα and SUG-1 at 60 min following RA addition.
Next, we aimed at investigating whether SUG-1 was also recruited to the promoter of other RA target genes such as the RARβ2 gene. In MCF7 cells and most breast cancer cell lines, the RARβ2 gene is silenced by DNA methylation (31) and is not expressed in response to RA (data not shown). However, the proximal promoter region, which contains the DR5 RARE (Fig. 3G), is accessible to transcription factors, and ChIP experiments (Fig. 3G) gave results that were very similar to those obtained above for the Cyp26A1 promoter.
Altogether these results indicate that SUG-1 is recruited concomitantly with SRC-3 to RARα target gene promoters, further supporting the idea that SUG-1 is involved in transcription. Note that a weak earlier (20 min after RA addition) peak was also observed (Fig. 3, B, C, and G), suggesting the existence of sequential waves of promoter accessibility for SUG-1 and SRC-3 (32).
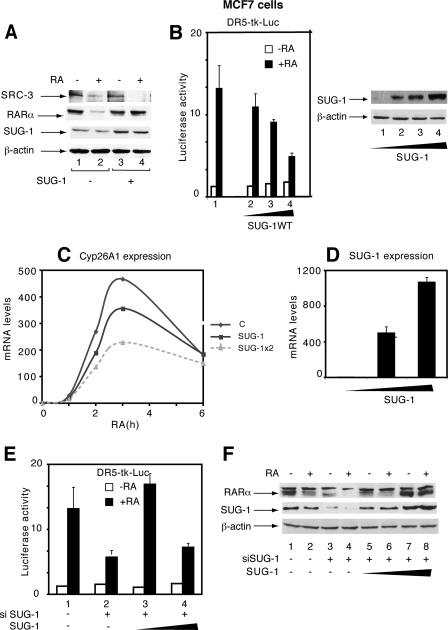
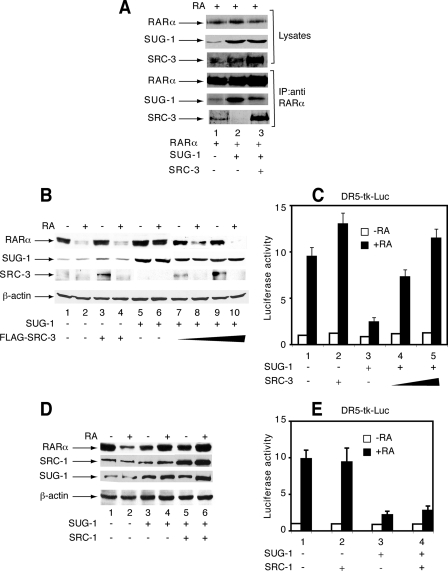
Overexpression of SUG-1 Blocks Both RA-induced Expression of RARα-Target Genes and RARα Degradation—We analyzed the influence of SUG-1 overexpression on SRC-3 degradation and the transcription of RARα target genes. Overexpression of SUG-1 in MCF7 cells increased the efficiency of SRC-3 degradation (Fig. 4A, lane 4) confirming the contribution of SUG-1 in this process. Unexpectedly, forced expression of SUG-1 reduced the RA-induced expression of a DR5-driven Luciferase reporter gene (Fig. 4B) and of the Cyp26A1 endogenous target gene (Fig. 4, C and D). It also inhibited the RA-induced degradation of RARα (Fig. 4A). Such effects were specific for SUG-1, because overexpression of SRC-3 or the other p160 coactivators did not affect the transcription of RA target genes or the degradation of RARα (20) (see also Fig. 6 below). Interestingly, in MCF7 cell knockdown for SUG-1, re-expression of SUG-1 to endogenous levels restored both transcription (Fig. 4E, lanes 2 and 3) and RARα degradation (Fig. 4F, lanes 4 and 6). However, re-expression of SUG-1 to excess amounts had an inhibitory effect on both processes (Fig. 4E, lane 4 and Fig. 4F, lane 8). Collectively these results suggest that, when present in excess amounts, SUG-1 competes with endogenous complexes involved in the transcription of RARα target genes and in the degradation of RARα.
FIGURE 4.
In MCF7 cells, overexpression of SUG-1 blocks the transcription of RARα target genes and the degradation of RARα. A, MCF7 cells were transfected with a vector encoding SUG-1 and were RA-treated. SRC-3 and RARα degradation as well as the efficiency of SUG-1 overexpression were analyzed by immunoblotting. B, overexpression of SUG-1 blocks the RA-induced expression of a DR5-tk luciferase reporter gene. The values are the mean ± S.D. of triplicate experiments. The efficiency of SUG-1 overexpression is shown in the right panel. C and D, overexpression of SUG-1 reduces the RA-induced expression of Cyp26A1, as assessed by qRT-PCR. The results are the average of three experiments that agreed within 15%. E and F, in MCF7 cell knockdown for SUG-1, re-expression of SUG-1 in excess amounts blocks the degradation of RARα and the expression of a DR5-tk-Luc reporter gene. The values are the mean ± S.D. of three independent experiments.
FIGURE 6.
Overexpression of SRC-3 reverses the inhibitory effects of SUG-1 on RARα degradation and RARα-mediated transcription. A, in COS-1 cells cotransfected with RARα and SUG-1 expression vectors and RA-treated for 1 h, overexpression of SRC-3 reverses the interaction of SUG-1 with RARα. Nuclear extracts were immunoprecipitated with RARα monoclonal antibodies and analyzed by immunoblotting. The upper panels correspond to aliquots of unprecipitated extracts. B and C, COS-1 cells were cotransfected with the RARα vector and the DR5-tk-LUC reporter construct, along with SUG-1 and/or FLAG-SRC-3 and RA-treated. Extracts were analyzed by immunoblotting (B) and for luciferase activity (C). The results are the mean ± S.D. of at least three independent experiments. D and E, the same as in B and C but with SRC-1 instead of FLAG-SRC-3.
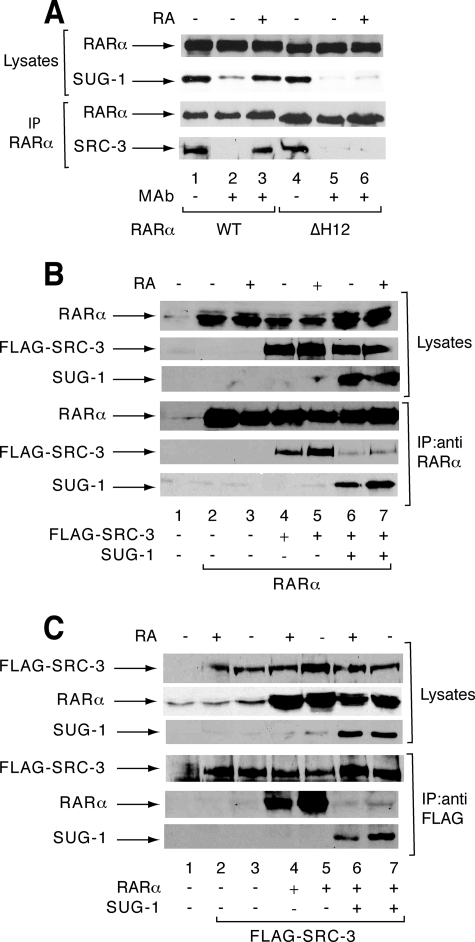
Overexpressed SUG-1 Interferes with SRC-3 for Binding to RARα—In response to RA, SRC-3 and several coregulators interact with RARα at the coactivator surface involving helix 12 (3) (Fig. 5A). Interestingly, SUG-1 interacts with this same RARα surface (22) (Fig. 5A), the integrity of which is required for both RARα transcriptional activity and degradation (3, 18).
FIGURE 5.
SUG-1 interferes with SRC-3 for binding to RARα. A, SUG-1 and SRC-3 interact with the same helix 12 of RARα. Nuclear extracts from COS-1 cells transfected with an expression vector for RARα (WT or ΔH12) along with SUG-1 or FLAG-SRC-3 were RA-treated (1 h) and immunoprecipitated with RARα monoclonal antibodies, followed by immunoblotting. Lanes 1 and 4 correspond to 5% of the amount of immunoprecipitated extracts. B and C, overexpression of SUG-1 blocks the interaction of RARα with FLAG-SRC-3 in cotransfected COS cells. Coimmunoprecipitations were performed with either RARα (B) or FLAG (C) antibodies and analyzed by immunoblotting. The upper panels correspond to aliquots (10%) of unprecipitated extracts.
This prompted us to investigate whether overexpressed SUG-1 would interfere with SRC-3 for RARα binding. Coimmunoprecipitations were performed with extracts from COS-1 cells cotransfected with RARα, FLAG-SRC-3, and/or SUG-1 vectors. In the absence of SUG-1, an interaction between RARα and SRC-3 was detected in response to RA whatever immunoprecipitation was performed with RARα (Fig. 5B, lanes 4 and 5) or FLAG antibodies (Fig. 5C, lanes 4 and 5). When SUG-1 was overexpressed, this interaction was decreased (Fig. 5, D and E, lanes 6 and 7). Thus, when present in high amounts, SUG-1 interferes with the interaction between RARα and SRC-3.
Next we investigated whether, reciprocally, overexpression of SRC-3 might squelch the excess of SUG-1 and thereby restore the interaction between RARα and SRC-3. We found that in cells cotransfected with the RARα and SUG-1 vectors, the interaction between RARα and SUG-1 was decreased upon forced expression of SRC-3 (Fig. 6A). Subsequently the interaction between RARα and SRC-3 was restored (Fig. 6A). RARα degradation and transcriptional activity, which were blocked in the presence of an excess of SUG-1 (Fig. 6B, lane 6 and Fig. 6C, lane 3), were also restored upon overexpression of SRC-3 (Fig. 6B, lanes 7-10 and Fig. 6C, lanes 4 and 5). In contrast, SRC-1, which does not interact with SUG-1, was ineffective (Fig. 6, D and E). Altogether these results converge to the conclusion that SRC-3 would specifically squelch the excess of SUG-1 through its ability to bind SUG-1 and/or RARα, thereby restoring the ability of RARα to activate transcription and to be degraded.
DISCUSSION
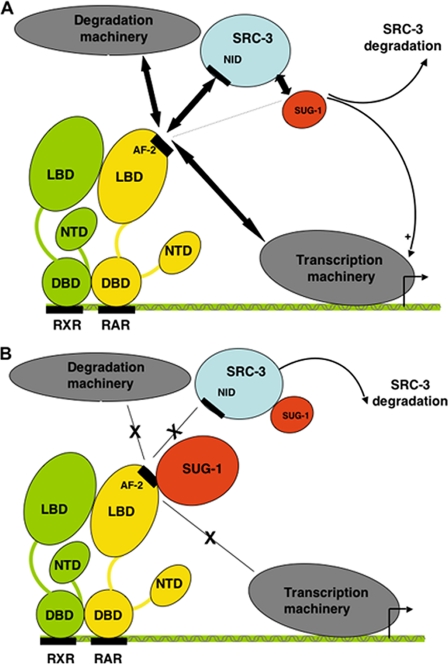
In eukaryotic cells, gene expression in response to RA is a rapid process that is rigorously controlled by an ever-growing network of dynamic and carefully orchestrated series of exchanges between RARs and coregulatory proteins (21). Indeed, in response to RA, coactivators of the p160 SRC family are recruited to cognate gene promoters by RARs through direct contacts involving their nuclear receptor interaction domain and transmit the activation signal through their AD1 and AD2 domains, which serve as platforms to recruit other factors that contribute to transcriptional activation. In addition, a variety of post-translational modifications and degradation processes cooperate with the ligand to fine-tune the transcription of RA target genes (11, 21). Here we identified a new mechanism underlying the activation of RARα target genes, which involves SUG-1, one of the ATPases of the 19 S regulatory complex of the 26 S proteasome (Fig. 7).
FIGURE 7.
Model recapitulating the roles played by SUG-1 in the control of RARα target genes. A, in response to RA, SUG-1 interacts with SRC-3 and thereby fine-tunes RARα-mediated transcription. It also contributes to the proteasomal degradation of SRC-3. B, when present in excess amounts, SUG-1 accelerates the degradation of SRC-3. It also blocks the AF-2 domain of RARα and impedes the recruitment of SRC-3 and of the transcription and degradation machineries.
SUG-1 Interacts with SRC-3 and Contributes to Its Degradation—Given that, in response to RA, RARα as well as its coactivator SRC-3 are degraded by the 26 S proteasome (18, 20), we investigated whether SUG-1 is involved in these processes. We have shown that SUG-1 interacts with SRC-3 both in vitro and in vivo and contributes to the proteasomal degradation of SRC-3. The exact mechanism of this effect is still unclear, but may relate to the chaperone-like activity of SUG-1 such that SRC-3 degradation is favored (16). However, one cannot exclude that SUG-1 also participates in the assembly of the 19 S and 20 S subcomplexes to shuttle SRC-3 to the 20 S core for degradation (33, 34). In contrast, according to our data, SUG-1 is not required for the degradation of RARα. SRC-3 is not required either (Fig. 2C), in contrast with what was reported for estrogen receptor α (35), suggesting again a degree of specificity. Further investigations will be necessary to decipher how the proteasome is recruited for RARα degradation.
SUG-1 Participates in RA Target Gene Transcription—The present study indicates that SUG-1 also participates in the transcription of RARα target genes. According to the new arising concept that dynamic exchanges of coactivators are required for transcription to proceed, SUG-1 might play a role in the control of RARα-mediated transcription through its ability to promote the degradation of SRC-3. Such a process might serve to clear out SRC-3 so that other coregulators can subsequently bind to facilitate the recruitment of the transcriptional machinery (15, 36). However, such a model is unlikely because SRC-3 degradation occurs rather late after RA addition (10-16 h) when transcription has declined (see Fig. 2) (20). Thus SUG-1-mediated SRC-3 degradation would rather signal the end of the transcriptional process (37).
In fact, the present study presents evidence that, in vivo, SUG-1 is rapidly (within 1 h) recruited onto promoters of RARα target genes. Pertinent with this new finding, SUG-1 has been recently reported to associate with several yeast promoters (38-40), the Adenovirus early region promoters (41), estrogen receptor-regulated promoters (32, 42), and several other active mammalian promoters (25, 26). Given that SUG-1 interacts with SRC-3 and is recruited concomitantly with SRC-3, one can propose that SUG-1 might be a positive coregulator assembling several complexes at RARα target genes as already described for the (Spt-Ada-Gen5-acetyltransferase) histone-acetyl-transferase complex (43). However it cannot be excluded that SUG-1 is also recruited to histones as a component of the 19 S or the (ATPases independent of 20 S) complexes and as such reconfigures local chromatin to promote recruitment of appropriate histone modifiers (44, 45).
Whatever the mechanism of SUG-1 action may be, it is interesting to note that in fine SUG-1 has an impact on RA-induced cellular differentiation. Indeed, taking as a model the well known NB4 cells, which differentiate into granulo-cytes in response to RA, we found that siRNA-mediated knockdown of SUG-1 decreases the RA-induced expression of several differentiation markers (supplemental Fig. S1).
When Present in Excess Amounts, SUG-1 Blocks Both RARα Degradation and RARα-mediated Transcription—Unexpectedly the present study demonstrates that SUG-1 overexpression inhibits the transcription of RARα target genes as well as RARα degradation. The exact mechanism of this inhibition is not yet clear, but several hypotheses might be proposed (Fig. 7). First, a radical one might be suggested in which this inhibition would reflect the increased degradation of SRC-3 observed upon SUG-1 overexpression. Although consistent with the inhibition of RARα-mediated transcription, such a model does not fit with the inhibition of RARα degradation, because this process does not depend on SRC-3. Second, because SUG-1 interacts with several transcription factors (22, 40, 46), the observed inhibition may reflect a transcriptional interference/squelching phenomenon, as previously observed for other components of transcriptional multiprotein complexes. In this regard, high levels of SUG-1 have been shown to interfere with endogenous SUG-1 function in proteasomal-mediated protein degradation and hence activation of E1A (41), VP16 (47), and RARγ2 (17).
In fact, because SUG-1 is able to interact with RARα at the same surface involving H12 that is required for the recruitment of coactivators, the present study rather indicates that overexpressed SUG-1 binds RARα, which as such becomes stalled, unable to recruit the adequate coregulators involved in transcription or in degradation (Fig. 7B). Because the blocking effect of SUG-1 can be overcome by increasing the amounts of SRC-3, the ratio between RARα, SRC-3, and SUG-1 appears to be crucial for the control of RARα degradation and transcriptional activity, in line with their ability to interact with each other.
In conclusion, consistent with the dynamics of the association-dissociation of coregulators involved in RARα-mediated transcription, we can suggest that SUG-1 shuttles between different complexes so that the correct proteins are present with the right activity at the right place and the right time. At the end, SUG-1 would join proteolytic complexes to promote the degradation of SRC-3 and the end of the RA signal. Further investigations will be required to characterize the SUG-1-associated complexes and how they participate in the proteolytic and non-proteolytic processes. Nevertheless our results demonstrate that SUG-1 plays a unique role in the expression of RA target genes by providing a link between transcription and degradation.
Supplementary Material
Acknowledgments
We thank R. Losson (Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC)) for providing the SUG-1 constructs and members of the cell culture facilities (IGBMC) for help.
This work was supported in part by funds from CNRS, INSERM, the Agence Nationale pour la Recherche (ANR-05-BLAN-0390-02), the Institut National du Cancer (INCa PL06-095 and PL07-96099), the Association pour la Recherche sur le Cancer (ARC A05/2/3139), the Associazione Italiana per la Ricerca contro il Cancro, the Istituto Superiore di Sanità, the Progetto Final-izzato Oncologia (CNR and Ministero dell'Università e della Ricerca Scientifica), and the Fondo D'Investimento per la Ricerca Biotecnologica. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: RA, retinoic acid; RAR, RA receptor; RXR, retinoid X receptor; SRC, steroid receptor coactivator; GST, glutathione S-transferase; ChIP, chromatin immunoprecipitation; siRNA, small interfering RNA; Re-ChIP, sequential ChIP; RT, reverse transcription; qRT, quantitative RT; RARE, RA response element.
References
- 1.Germain, P., Staels, B., Dacquet, C., Spedding, M., and Laudet, V. (2006) Pharmacol. Rev. 58 685-704 [DOI] [PubMed] [Google Scholar]
- 2.Laudet, V., and Gronemeyer, H. (2001) Nuclear Receptor Factsbook, Academic Press, London
- 3.Chambon, P. (1996) FASEB J. 10 940-954 [PubMed] [Google Scholar]
- 4.Glass, C. K., and Rosenfeld, M. G. (2000) Genes Dev. 14 121-141 [PubMed] [Google Scholar]
- 5.Lefebvre, P., Martin, P. J., Flajollet, S., Dedieu, S., Billaut, X., and Lefebvre, B. (2005) Vitam. Horm. 70 199-264 [DOI] [PubMed] [Google Scholar]
- 6.Rochette-Egly, C. (2005) J. Biol. Chem. 280 32565-32568 [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld, M. G., Lunyak, V. V., and Glass, C. K. (2006) Genes Dev. 20 1405-1428 [DOI] [PubMed] [Google Scholar]
- 8.Dilworth, F. J., and Chambon, P. (2001) Oncogene 20 3047-3054 [DOI] [PubMed] [Google Scholar]
- 9.Lonard, D. M., and O'Malley, B. W. (2005) Trends Biochem. Sci. 30 126-132 [DOI] [PubMed] [Google Scholar]
- 10.Reid, G., Hubner, M. R., Metivier, R., Brand, H., Denger, S., Manu, D., Beaudouin, J., Ellenberg, J., and Gannon, F. (2003) Mol. Cell 11 695-707 [DOI] [PubMed] [Google Scholar]
- 11.Bastien, J., and Rochette-Egly, C. (2004) Gene (Amst.) 328 1-16 [DOI] [PubMed] [Google Scholar]
- 12.Kinyamu, H. K., Chen, J., and Archer, T. K. (2005) J. Mol. Endocrinol. 34 281-297 [DOI] [PubMed] [Google Scholar]
- 13.Pickart, C. M., and Cohen, R. E. (2004) Nat. Rev. Mol. Cell. Biol. 5 177-187 [DOI] [PubMed] [Google Scholar]
- 14.Muratani, M., and Tansey, W. P. (2003) Nat. Rev. Mol. Cell. Biol. 4 192-201 [DOI] [PubMed] [Google Scholar]
- 15.Collins, G. A., and Tansey, W. P. (2006) Curr. Opin. Genet. Dev. 16 197-202 [DOI] [PubMed] [Google Scholar]
- 16.Demartino, G. N., and Gillette, T. G. (2007) Cell 129 659-662 [DOI] [PubMed] [Google Scholar]
- 17.Gianni, M., Bauer, A., Garattini, E., Chambon, P., and Rochette-Egly, C. (2002) EMBO J. 21 3760-3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopf, E., Plassat, J. L., Vivat, V., de The, H., Chambon, P., and Rochette-Egly, C. (2000) J. Biol. Chem. 275 33280-33288 [DOI] [PubMed] [Google Scholar]
- 19.Perissi, V., Scafoglio, C., Zhang, J., Ohgi, K. A., Rose, D. W., Glass, C. K., and Rosenfeld, M. G. (2008) Mol. Cell 29 755-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianni, M., Parrella, E., Raska, I., Gaillard, E., Nigro, E. A., Gaudon, C., Garattini, E., and Rochette-Egly, C. (2006) EMBO J. 25 739-751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bour, G., Lalevee, S., and Rochette-Egly, C. (2007) Trends Cell Biol. 17 302-309 [DOI] [PubMed] [Google Scholar]
- 22.vom Baur, E., Zechel, C., Heery, D., Heine, M. J., Garnier, J. M., Vivat, V., Le Douarin, B., Gronemeyer, H., Chambon, P., and Losson, R. (1996) EMBO J. 15 110-124 [PMC free article] [PubMed] [Google Scholar]
- 23.Bour, G., Plassat, J. L., Bauer, A., Lalevee, S., and Rochette-Egly, C. (2005) J. Biol. Chem. 280 17027-17037 [DOI] [PubMed] [Google Scholar]
- 24.Das, P. M., Ramachandran, K., vanWert, J., and Singal, R. (2004) BioTechniques 37 961-969 [DOI] [PubMed] [Google Scholar]
- 25.Bhat, K. P., Turner, J. D., Myers, S. E., Cape, A. D., Ting, J. P., and Greer, S. F. (2008) Mol. Immunol. 45 2214-2224 [DOI] [PubMed] [Google Scholar]
- 26.Zhu, Q., Wani, G., Yao, J., Patnaik, S., Wang, Q. E., El-Mahdy, M. A., Praetorius-Ibba, M., and Wani, A. A. (2007) Oncogene 26 4199-4208 [DOI] [PubMed] [Google Scholar]
- 27.Brown, K., Chen, Y., Underhill, T. M., Mymryk, J. S., and Torchia, J. (2003) J. Biol. Chem. 278 39402-39412 [DOI] [PubMed] [Google Scholar]
- 28.Zhang, H., Yi, X., Sun, X., Yin, N., Shi, B., Wu, H., Wang, D., Wu, G., and Shang, Y. (2004) Genes Dev. 18 1753-1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loudig, O., Maclean, G. A., Dore, N. L., Luu, L., and Petkovich, M. (2005) Biochem. J. 392 241-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruck, N., Vitoux, D., Ferry, C., Duong, V., Bauer, A., de The, H., and Rochette-Egly, C. (2009) EMBO J. 28 34-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widschwendter, M., Berger, J., Hermann, M., Muller, H. M., Amberger, A., Zeschnigk, M., Widschwendter, A., Abendstein, B., Zeimet, A. G., Daxenbichler, G., and Marth, C. (2000) J. Natl. Cancer Inst. 92 826-832 [DOI] [PubMed] [Google Scholar]
- 32.Metivier, R., Penot, G., Hubner, M. R., Reid, G., Brand, H., Kos, M., and Gannon, F. (2003) Cell 115 751-763 [DOI] [PubMed] [Google Scholar]
- 33.Lassot, I., Latreille, D., Rousset, E., Sourisseau, M., Linares, L. K., Chable-Bessia, C., Coux, O., Benkirane, M., and Kiernan, R. E. (2007) Mol. Cell 25 369-383 [DOI] [PubMed] [Google Scholar]
- 34.Satoh, K., Sasajima, H., Nyoumura, K. I., Yokosawa, H., and Sawada, H. (2001) Biochemistry 40 314-319 [DOI] [PubMed] [Google Scholar]
- 35.Shao, W., Keeton, E. K., McDonnell, D. P., and Brown, M. (2004) Proc. Natl. Acad. Sci. U. S. A 101 11599-11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennis, A. P., and O'Malley, B., W. (2005) J. Steroid Biochem. Mol. Biol. 93 139-151 [DOI] [PubMed] [Google Scholar]
- 37.Tansey, W. P. (2001) Genes Dev. 15 1045-1050 [DOI] [PubMed] [Google Scholar]
- 38.Auld, K. L., Brown, C. R., Casolari, J. M., Komili, S., and Silver, P. A. (2006) Mol. Cell 21 861-871 [DOI] [PubMed] [Google Scholar]
- 39.Morris, M. C., Kaiser, P., Rudyak, S., Baskerville, C., Watson, M. H., and Reed, S. I. (2003) Nature 423 1009-1013 [DOI] [PubMed] [Google Scholar]
- 40.Gillette, T. G., Gonzalez, F., Delahodde, A., Johnston, S. A., and Kodadek, T. (2004) Proc. Natl. Acad. Sci. U. S. A 101 5904-5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasti, M., Grand, R. J., Yousef, A. F., Shuen, M., Mymryk, J. S., Gallimore, P. H., and Turnell, A. S. (2006) EMBO J. 25 2710-2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, H., Sun, L., Liang, J., Yu, W., Zhang, Y., Wang, Y., Chen, Y., Li, R., Sun, X., and Shang, Y. (2006) EMBO J. 25 4223-4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, D., Ezhkova, E., Li, B., Pattenden, S. G., Tansey, W. P., and Workman, J. L. (2005) Cell 123 423-436 [DOI] [PubMed] [Google Scholar]
- 44.Ezhkova, E., and Tansey, W. P. (2004) Mol. Cell 13 435-442 [DOI] [PubMed] [Google Scholar]
- 45.Koues, O. I., Dudley, R. K., Truax, A. D., Gerhardt, D., Bhat, K. P., McNeal, S., and Greer, S. F. (2008) Mol. Cell. Biol. 28 5837-5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weeda, G., Rossignol, M., Fraser, R. A., Winkler, G. S., Vermeulen, W., van't Veer, L. J., Ma, L., Hoeijmakers, J. H., and Egly, J. M. (1997) Nucleic Acids Res. 25 2274-2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu, Q., Yao, J., Wani, G., Chen, J., Wang, Q. E., and Wani, A. A. (2004) FEBS Lett. 556 19-25 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.