Abstract
A man presented with recurrent syncope, weakness and fatigue. His ECG showed marked QRS widening and he had gross hyponatraemia and hypokalaemia. His medications included bendroflumethiazide (long term) and flecainide (started 2 months previously).
This presentation was consistent with flacainide cardiotoxicity exacerbated by electrolyte disturbance. The syncopal episodes probably represented life‐threatening arrhythmias. The ECG and symptoms resolved completely once the electrolytes were corrected.
Increased cardiotoxicity with hypokalaemia is documented, but not widely recognised. Hyponatraemia‐induced flecainide cardiotoxicity has not been documented. The clinical effects of flecainide are due to use‐dependent block of sodium channels. There are reports that support the use of hypertonic sodium salts to reverse flecainide toxicity via antagonism at the receptor. By this rationale, hyponatraemia would lead to Flecainide toxicity.
Flecainide has been shown to reduce salt absorption in animal bowel. It is possible that in combination with bendroflumethiazide it acted synergistically to produce profound electrolyte disturbance.
Flecainide cardiotoxicity has a significant mortality and can present non‐specifically. Thus, early recognition is essential. This case demonstrates the importance of strict electrolyte control in patients who are on flecainide. We would discourage concomitant use of flecainide and bendroflumethiazide.
A 63‐year‐old man presented to the emergency department with syncopal episodes. These precipitated without warning and loss of consciousness lasting for seconds had been observed. There was prompt and spontaneous full recovery. He also described progressive fatigue and muscle weakness over recent weeks.
He had presented 2 months previously with a regular narrow complex tachycardia at 180 beats/min. This had cardioverted successfully with intravenous flecainide, and he was started on flecainide 100 mg twice daily orally as prophylaxis. Echocardiogram had confirmed a structurally normal heart and resting ECG was normal. There was no evidence of ischaemia on exercise testing. There had been no further episodes after discharge.
Other medical history included hypertension for which he took long‐term bendroflumethiazide 2.5 mg once daily and felodipine 2.5 mg once daily.
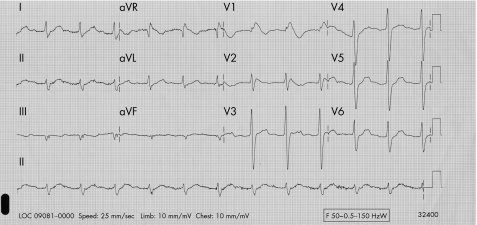
On this occasion, initial investigation revealed a blood pressure of 125/80 mm Hg with no postural deficit. The ECG showed sinus rhythm with marked QRS widening (fig 1). Blood tests of note were a serum sodium of 118 mmol and potassium of 2.7 mmol. There was no history of gastrointestinal loss or excessive fluid intake.
Figure 1 ECG showing sinus rhythm with marked QRS widening.
Bendroflumethiazide and flecainide were stopped and he was admitted under cardiologists for further investigations and management.
Discussion
This presentation is consistent with flecainide cardiotoxicity exacerbated by electrolyte disturbance. The syncopal episodes probably represented life‐threatening arrhythmias such as ventricular tachycardia. The ECG and symptoms resolved completely once the electrolytes were corrected.
Flecainide is a class 1c anti‐arrhythmic agent. It depresses the rate of depolarisation of action potentials producing a membrane‐stabilising action. The clinical effects of flecainide are due to use‐dependent block of sodium channels. It is a very effective anti‐arrhythmic agent but can lead to life‐threatening pro‐arrhythmogenic effects. In the Cardiac Arrhythmia Suppression Trial study an increased mortality after myocardial infarction was shown.1 Flecainide is therefore contraindicated in patients with structural heart disease.
In cases of overdose, the mortality with class 1c agents has been reported to approach 22%.2 Conduction disturbances can rapidly progress to life‐threatening arrhythmias, electromechanical dissociation and asystole. Therefore, early recognition and supportive treatment are paramount.
Increased flecainide cardiotoxicity with hypokalaemia is documented in the drug manufacturer's product characteristics. It is not widely recognised by clinicians and we were only able to find one supporting reference.3
Hyponatraemia‐induced flecainide cardiotoxicity has not been documented. Animal studies and anecdotal human reports support the use of hypertonic sodium salts to reverse flecainide toxicity in an emergency setting.4 The cellular mechanism is thought to involve antagonism of the interaction of flecainide with the sodium channel via sodium ions. By this rationale, low levels of sodium ions would predispose to flecainide toxicity.
Flecainide has also been shown to reduce transepithelial sodium and water absorption in animal studies (class 1 agents can cause diarrhoea). Our patient had been on long‐term bendroflumethiazide treatment without previous electrolyte imbalance. Within 2 months of starting flecainide the electrolyte levels had dropped rapidly. This raises the possibility that flecainide can act synergistically with bendroflumethiazide to exacerbate profound electrolyte loss.
Flecainide cardiotoxicity has a significant mortality, and can present non‐specifically.
This case illustrates the importance of correcting electrolyte disturbances before starting flecainide treatment and monitoring electrolytes in high‐risk individuals. We would discourage concomitant bendroflumethiazide and flecainide use.
Footnotes
Competing interests: None.
Informed consent was obtained from the patient for publication of their details in this paper.
References
- 1.The Cardiac Arrhythmia Suppression Trial (CAST) Investigators Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989321406–412. [DOI] [PubMed] [Google Scholar]
- 2.Koppel C, Oberdisse U, Heinemeyer G. Clinical course and outcome in class Ic antiarrhythmic overdose. J Toxicol Clin Toxicol 199028433–444. [DOI] [PubMed] [Google Scholar]
- 3.Ohki R, Takahashi M, Mizuno O.et al Torsades de pointes ventricular tachycardia induced by mosapride and flecainide in the presence of hypokalemia. Pacing Clin Electrophysiol 200124119–121. [DOI] [PubMed] [Google Scholar]
- 4.Golman M J, Mowry J B, Kirk M A. Sodium Bicarbonate to correct widened QRS in a case of flecainide overdose. J Emerg Med 199715183–186. [DOI] [PubMed] [Google Scholar]