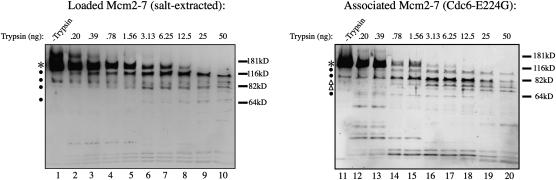
Figure 4.
Loaded and associated Mcm2–7 complexes are conformationally distinct. Limited trypsin digestion of loaded and associated Mcm2–7. To isolate loaded Mcm2–7, assembled pre-RCs were washed with high-salt buffer. To enrich for associated Mcm2–7, pre-RCs were assembled using Cdc6-E224G. The resulting Mcm2–7 complexes were washed with buffer compatible with trypsin and treated with the indicated amounts of TPCK-treated trypsin for 25 min at 25°C. Samples were analyzed by SDS-PAGE followed by immunoblotting for the Mcm2–7 complexes. (*) Full-length Mcm2–7 proteins; (●) degradation intermediates present in both proteolysis profiles; (△) degradation intermediates present in the proteolysis profiles of the associated Mcm2–7 complexes but not in that of the loaded Mcm2–7 complexes. Note that the use of a 4%–20% polyacrylamide gel resulted in the intact Mcm2–7 proteins migrating as a single broad band.