Abstract
Nausea and vomiting are amongst the most common symptoms encountered in medicine as either symptoms of diseases or side effects of treatments. In a more biological setting they are also important components of an organism’s defences against ingested toxins. Identification of treatments for nausea and vomiting and reduction of emetic liability of new therapies has largely relied on the use of animal models, and although such models have proven invaluable in identification of the anti-emetic effects of both 5-hydroxytryptamine3 and neurokinin1 receptor antagonists selection of appropriate models is still a matter of debate. The present paper focuses on a number of controversial issues and gaps in our knowledge in the study of the physiology of nausea and vomiting including: The choice of species for the study of emesis and the underlying behavioural (e.g. neophobia), anatomical (e.g. elongated, narrow abdominal oesophagus with reduced ability to shorten) and physiological (e.g. brainstem circuitry) mechanisms that explain the lack of a vomiting reflex in certain species (e.g. rats); The choice of response to measure (emesis[retching and vomiting], conditioned flavour avoidance or aversion, ingestion of clay[pica], plasma hormone levels[e.g. vasopressin], gastric dysrhythmias) and the relationship of these responses to those observed in humans and especially to the sensation of nausea; The stimulus coding of nausea and emesis by abdominal visceral afferents and especially the vagus—how do the afferents encode information for normal postprandial sensations, nausea and finally vomiting?; Understanding the central processing of signals for nausea and vomiting is particularly problematic in the light of observations that vomiting is more readily amenable to pharmacological treatment than is nausea, despite the assumption that nausea represents “low” intensity activation of pathways that can evoke vomiting when stimulated more intensely.
Keywords: Nausea, Vomiting, Vagus, Pica, Flavour aversion, Conditioned taste aversion Contents
1. Introduction
Nausea and vomiting are amongst the most common symptoms encountered in medicine. They occur separately and together in a diverse range of diseases, following anaesthesia and surgery, and are side effects of both current drug treatments and novel therapies in development where there may be dose limiting toxicities (for review, see Rudd and Andrews, 2005). Nausea and vomiting are also components of the body’s defensive response to toxins accidentally ingested with food. Understanding the pathways by which the sensation of nausea is generated and reflex vomiting is evoked is essential to identifying novel pharmacological approaches to the development of antiemetics and also for gaining insights into the underlying defect in disorders where nausea and vomiting are cardinal features, such as cyclic vomiting syndrome where patients may have a median peak intensity of 6 vomits an hour at the height of an attack (Li and Fleisher, 1999). The investigation of pathways and their pharmacology relies to a large extent upon the use of appropriate animal models and this paper will focus on some of the issues related to the selection of models for the investigation of nausea and vomiting and also on the limitations of using species without an emetic reflex (e.g. rats) to study malaise originating in the upper gut.
In addition, research into nausea and vomiting provides important insights into information processing in the autonomic nervous system. For example, the abdominal vagal afferent fibres can evoke both responses, the nucleus tractus solitarius (NTS) plays a major role in integrating the emetic response and is a target for anti-emetic agents, and the autonomic efferents mediate many of the physiological changes that accompany both nausea (e.g., cutaneous vasoconstriction and sweating) and vomiting (e.g., proximal stomach relaxation, retrograde giant contraction of the small intestine). Furthermore, because some motor components (e.g. inhibition of the crural diaphragm) of the emetic reflex occur in other reflexes, such as belching, and diseases, for example gastro-oesophageal reflux disease, the study of emesis may provide fortuitous insights into the pathophysiology and treatment of other disorders. In this context it is important to note that several “agonist” antiemetics are also effective in gastroesophageal reflux disease or models (e.g., the opioid receptor agonist morphine; cannabinoid CB1 receptor agonists, GABA B receptor agonists (Penagini and Bianchi, 1997; Holloway, 2001; Tonini et al., 2004).
2. Choice of species in nausea and emesis research
Vertebrates (fish, amphibia, reptiles, birds and mammals) have significant species differences in the mechanisms and especially the mechanics of vomiting that make it difficult to make more than general comparisons between animals. Even amongst the mammals it may be difficult to extrapolate findings from laboratory animal studies to explain nausea and vomiting in humans, especially as we begin to understand more of the species differences between neurotransmitter receptors and the way that they interact with synthetic ligands (e.g., neurokinin NK1 receptors; Andrews and Rudd, 2004). Emesis (retching and vomiting), which can be overtly measured in some laboratory animals, is much easier to study and interpret than behaviours that have been associated with the subjective experience of nausea. However, even with research on vomiting there are important species differences.
2.1. The vomiting reflex is lacking in some species
In general, the vomiting reflex is arguably not essential for survival; but it is advantageous for ridding the body of ingested toxins and may prove particularly useful for a species in a specific niche. Several mammalian species commonly used in laboratory research, including the rat, mouse, hamster, guinea pig, and rabbit, appear not to have a vomiting reflex. Although a large number of toxicosis studies have been conducted in the rat, a relatively limited number of stimuli, including, gastrointestinal irritants, motion, apomorphine, and cytotoxic drugs, have been used to study behaviours associated with malaise. While there is little doubt that overt emesis has not been observed in the laboratory rat, only a few strains (e.g., Wistar) have been studied, and this may be significant because it is known that conditioned flavour avoidances are variably induced in different strains of mice (e.g., Risinger and Brown, 1996; Risinger and Cunningham, 2000; Bielavska et al., 2002). Furthermore, there are isolated reports of “retching” but not vomiting in mice, which if replicated may suggest that they have a degenerate reflex rather than an absent one (e.g., Furukawa and Yamada, 1980). Rats do have a gag reflex triggered by mechanical stimulation of the pharynx (Andrew, 1956) and a gag has features that could be considered similar to a single retch. This observation provides some evidence that the emetic reflex is degenerate in rodents. Some potentially emetic stimuli have been used in mice, hamsters, and rabbits to induce conditioned flavour avoidance or pica (measures of malaise; see below) and have failed to induce emesis (e.g., Hobbs et al., 1976; Fox, 1977; Santucci et al., 2000; Christian et al., 2001; Santucci et al., 2002; Yamamoto et al., 2002a). However, before stating that all rodents and lagomorphs lack an emetic reflex it may be wise to undertake further formal studies of this phenomenon using a wide range of doses and stimuli. As an alternative to emesis, the rat and other non-vomiting species probably use rapidly learned aversions to avoid toxicosis (see below). This is supported by the “nibbling” type of food intake in these species and some indications that they are neophobic (avoiding novel foods or tasting a small amount and returning later to ingest more if illness does not occur) (Mitchell, 1976). Both behaviours would serve to limit the consumption of a potentially poisonous meal. Furthermore, the rat and mouse possess more hepatic genes involved in detoxification than the human, and therefore the emetic reflex might provide less advantage in these species (Gibbs et al., 2004). The Rodentia are a very successful Order adapting to a variety of habitats and they account for >40% of all mammalian species (Dawkins, 2004), which provides support for the comment that the vomiting reflex is not essential for survival.
Although apparently absent in rodents and lagomorphs,the vomiting reflex is present in representatives of severalmammalian Orders, including Primates (e.g. human, Cynomologousmonkey, marmoset), Carnivora (dog, cat, ferret), Cetartiodactyla (e.g. pig, sperm whale), and Insectivora (e.g., house musk shrew, Suncus murinus), and it is also present throughout the Vertebrates, being demonstrated in prototypical fish, amphibia, reptiles and birds (Andrews et al., 1990, 2000; King, 1990; Sims et al., 2000; Tanihata et al., 2004). A discussion of the vomiting mechanisms in these non-mammalian species is beyond the scope of the current paper.
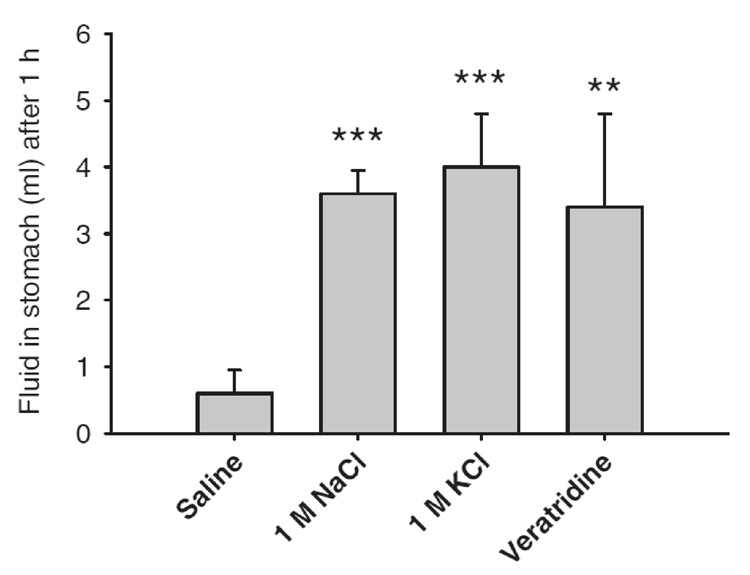
There are anatomical and neural circuitry differences between vomiting and non-vomiting mammalian species that would make vomiting difficult for non-emetic species even if they attempted to. The abdominal oesophagus is relatively wider and shorter in emetic species (Andrews, 1995). In addition, a comparison of the mouse and Suncus revealed that in the non-vomiting mouse the vagus had little ability to shorten the oesophagus longitudinally and hence educe one of the resistances to the flow of vomitus (Andrews et al., 2003). In general, the diaphragm appears to be more muscular in the emetic species so far studied with a reduced area of central tendon but this is not a universal finding (Andrews, unpublished observations) and the non-emetic roles of the diaphragm (e.g. breathing, coughing, sneezing, belching, hiccupping) make the significanceof this observation difficult to link to emesis. Taken overall these structural characteristics probably provide greater propulsive power and less resistance for expelling vomitus. Furthermore, the brainstem circuitry, e.g., medial medullary reticular neurons that provide input to phrenic motor neurons, that might be important for the production of emesis appear to be absent in the rat compared to the ferret, an emetic species (Dobbins and Feldman, 1994; Yates et al.,1999). It is worth noting that rats, like emetic species, have an area postrema (AP) but it is difficult to assign specific functions to this brain region because lesions of this site ablate vagal sensory neurons that terminate in the AP and often destroys some portion of the adjacent NTS, which receives a large vagal sensory projection from the gut (Norgren and Smith, 1988). The AP appears to be nonessential for the production of vomiting, e.g., motion can produce emesis in the cat and monkey after AP ablation(Fox et al., 1990) and gastric irritants (e.g., copper sulphate)can also induce emesis in the absence of the AP in the dog (Andrews et al., 2001). Other reflex neural circuitry of the brainstem appears to be similar between vomiting and nonvomiting species, e.g., the cardio-respiratory reflexes in rat versus Suncus (Paton et al., 2000; Smith et al., 2001b). It should also be noted that although rats do not vomit to intragastric stimuli that evoke emesis in susceptible species (e.g. ferret) (Andrews et al., 1990), rats do respond to stimuli such as veratridine and high concentrations of KCl and NaCl by markedly delaying gastric emptying (see Fig. 1). A delay in gastric emptying has been argued to be a component of the defensive response in rodents and in emetic species because this action would delay delivery of any toxin into the small intestine where it may produce more damage, especially if absorbed (Davis et al., 1986).
Fig. 1.
Effect of potentially emetic solutions on gastric emptying in the rat. Five millilitres of each solution was gavaged into the stomachs of rats previously deprived of food overnight (n = 5). ***p <0.0001 versus saline and **p <0.001 versus saline. (Andrews, Payne and Hawthorne, unpublished)
Among those animals that have a vomiting reflex there are several important issues that affect the interpretation of research findings across species. (1) There is no consistent profile across species for the rank order of sensitivity to different emetic stimuli. Although data are relatively limited in some species it is clear that the sensitivity profile to emetic stimuli is species dependent. For example, amongst species sensitive to apomorphine the rank order of emetic sensitivity is dog>human>ferret >cat (note: the cat requires doses in the mg/kg range in comparison to other species where the dose is Ag/kg), with Suncus and monkey being unresponsive. For the chemotherapeutic agent cisplatin the rank order is monkey>human>dog>ferret>cat>Suncus; and for total body X-radiation ferret > dog > human = monkey > cat (Andrews et al., 1990). Furthermore, the dog is sensitive to swinging-type motion whereas the cat is resistant (Noble, 1945) and it is argued that dogs appear to have approximately the same sensitivity to motion sickness as man (Money, 1970). Even amongst monkeys there are reports of sensitivity differences between squirrel and rhesus monkeys (Corcoran et al., 1990). (2) Not all emetic species are sensitive to all stimuli that cause vomiting. This is illustrated by Suncus, which unlike most vomiting species does not show emesis to morphine and apomorphine treatments (Selve et al., 1994). Apomorphine-induced emesis, although present in humans, is reported to be absent in the monkey (Brizzee et al., 1955) but present in another primate, the marmoset (Costall et al., 1987). (3) In some cases the function of emesis is different.
In most animals vomiting is a protective function that serves to expel toxins from the gastrointestinal tract prior to absorption, but in some species it is also used to rid the gut of indigestible material, such as bones from the guts of birds, hairballs from crocodiles and indigestible squid beaks in sharks (Darolova, 1991; Andrews et al., 2000), whilst vomiting is used as means of defence when Petrels are threatened. In addition, some species regurgitate partially digested food from the stomach to feed the young and relatives (e.g. wild dogs; Van Lawick-Goodall and Van Lawick-Goodall, 1970). Emesis might also serve as an anticipatory response generated by cognitive, visual, or flavour stimuli, and several categories of stimuli produce incidental activation of the emetic reflex, including chemotherapy, stress, intracranial pressure, and motion. Other behaviours also appear to be very similar to emesis. Although rumination and regurgitation are functionally different from vomiting and lack the propulsive power of emesis, the neural systems engaged in these different behaviours might be similar, e.g., in sheep (Carr et al., 1983).
2.2. What do conditioned flavour avoidance (CFA) and clay ingestion (pica) measure?
In species that do not possess a vomiting reflex, such as the rat, it is common to measure a conditioned flavour avoidance (CFA) or clay ingestion (pica; consumption of non-nutritive substances) as an index of malaise. It is tempting to be anthropomorphic and conclude that these measures reflect “nausea,” but we have no way of knowing whether this is true with reference to the human experience of nausea. We know that CFA and pica are associated with sickness and malaise, and appear to be additional strategies by which animals deal with toxicosis. Furthermore, unlike emesis, we lack an adequate understanding of the physiology of nausea and have little basis for comparing the behaviours of CFA and pica with reports of nausea in humans. However, investigations of the neural systems that produce CFA and pica might shed some light on the physiology of human nausea because these systems appear to have related neural circuitry and pharmacology, e.g., antiemetic drugs inhibit CFA and pica (e.g., Limebeer and Parker, 2000; Saeki et al., 2001; Rudd et al., 2002). Table 1 shows a list of species and stimuli that have been using to study emesis, CFA, and pica.
Table 1.
Mammalian specias and selected stimuli used to elicit emesis, conditioned flavour avoidance (CFA, and picaa
| Emesis | CFA | Pica | ||
|---|---|---|---|---|
| Cat | Cytotoxic drugs: | Cisplatin (McCarthy and Borison, 1984), Cyclophosphamide (Fetting et al., 1982) |
||
| Intragastric irritants: | Copper sulfate (Kayashima and Hyama, 1976) | |||
| Motion | (Crampton and Daunton, 1983) | (Fox et al., 1990) | ||
| Apomorphine | (Costello and Borison, 1977) | |||
| Morphine | (Villablanca et al., 1984) | |||
| Radiation | (Rabin et al., 1986b) | (Rabin et al., 1986b) | ||
| Nicotine | (Beleslin et al., 1981) | |||
| Hormones and neurotransmitters: | Angiotensin II (Rabin et al., 1986a) | |||
| Other: | Amphetamine (Rabin and Hunt, 1992) | |||
| Dog | Cytotoxic drugs: | Cisplatin (Gylys et al., 1979), Cyclophosphamide (Amber et al., 1990) |
||
| Intragastric irritants: | Ipecac (Gardner et al., 1996), Copper sulfate (Kayashima and Hayama, 1975) |
LiCl (Vavilova and Kassil, 1984) | ||
| Apomorphine | (Harrison et al., 1972) | |||
| Morphine | (Lefebvre et al., 1981) | |||
| Radiation | (Cooper and Mattsson, 1979) | |||
| Nicotine | (Vig, 1990) | |||
| Hormones and neurotransmitters: | CCK (Levine et al., 1984), Vasopressin (Wu et al., 1985), Peptide YY (Smith et al., 1989) |
|||
| Ferret | Cytotoxic drugs: | Cisplatin, cyclophosphamide (Andrews et al., 1990) | ||
| Intragastric irritants: | Copper sulfate, ipecac, NaCl, KCl (Andrews et al., 1990) | LiCl (Rabin and Hunt, 1992) | ||
| Apomorphine | (Andrews et al., 1990) | |||
| Morphine | (Barnes et al., 1991) | |||
| Radiation | (Andrews et al., 1990) | |||
| Guinea pig | Intragastric irritants: | LiCl (Braveman, 1974) | ||
| Hamster | Cytotoxic drugs: | Cyclophosphamide (Hobbs et al., 1976) | ||
| Intragastric irritants: | LiCl (Fox, 1977) | |||
| Apomorphine | (Nowlis et al., 1980) | |||
| Nicotine | (Etscorn et al., 1986) | |||
| Other: | 2-deoxy-d-glucose (Dibattista, 1988) | |||
| Human | Cytotoxic drugs: | Chemotherapy (Rudd and Andrews, 2005) | Chemotherapy (Schwartz et al., 1996) | |
| Intragastric irritants: | Ipecac (Jackson and Smith, 1978), Copper sulfate (Liu et al., 2001) |
Ipecac (Jackson and Smith, 1978) | ||
| Motion | (Yates et al., 1998) | (Arwas et al., 1989) | ||
| Apomorphine | (Schofferman, 1976) | |||
| Morphine | (Bailey et al., 1993) | |||
| Radiation | (Cordts et al., 1987) | (Carrell et al., 1986) | ||
| Hormones and neurotransmitters: | CCK (Miaskiewicz et al., 1989) | |||
| Other: | Pregnancy (Weigel and Weigel, 1989), Reduced intracranial pressure (Mokri, 2004) |
Pregnancy (Bayley et al., 2002) | Pregnancy (Corbett et al., 2003; Lopez et al., 2004), Gastric bypass surgery (Kushner et al., 2004) |
|
| Monkey | Cytotoxic drugs: | Cisplatin (Fukui et al., 1993) | Cyclophosphamide (Hikami et al., 1990) | |
| Intragastric irritants: | Copper sulfate (Fukui et al., 1993) | LiCl (Bergman and Glowa, 1986) | ||
| Motion | (Wilpizeski et al., 1987) | (Wilpizeski et al., 1987) | ||
| Radiation | (Brizzee, 1956) | |||
| Nicotine | (Spealman, 1983) | |||
| Hormones and neurotransmitters: | CCK (Perera et al., 1993) | |||
| Mouse | Cytotoxic drugs: | Cisplatin (Yamamoto et al., 2002a) | ||
| Intragastric irritants: | LiCl (Risinger and Cunningham, 2000) | |||
| Motion | (Kinney et al., 1993) | (Santucci et al., 2002) | ||
| Radiation | (Kimeldorf et al., 1960) | |||
| Nicotine | (Etscorn, 1980) | |||
| Other: | Magnetic field (Lockwood et al., 2003) | |||
| Rat | Cytotoxic drugs: | Cisplatin (Rudd et al., 1998), Cyclophosphamide (Ader, 1976) |
Cisplatin (Takeda et al., 1993) | |
| Intragastric irritants: | Ipecac (Rudd et al., 1998), Copper sulfate (Coil et al., 1978), LiCl (Coil et al., 1978) |
LiCl, Copper sulfate (Hasegawa et al., 1992) | ||
| Motion | (Hutchison, Jr., 1973) | (Mitchell et al., 1977a) | ||
| Apomorphine | (Krane et al., 1976) | (Hasegawa et al., 1992) | ||
| Morphine | (Aung et al., 2004) | |||
| Radiation | (Garcia et al., 1955) | (Yamamoto et al., 2002b) | ||
| Nicotine | (Etscorn et al., 1987) | |||
| Hormones and neurotransmitters: | NPY (Sipols et al., 1992), CCK (Ervin et al., 1995) |
NPY (Woods et al., 1998), CCK (McCutcheon et al., 1992) |
||
| Other: | Fat oxidation inhibitors (Singer et al., 1999), 2-deoxy-d-glucose (Stephan et al., 1999), Magnetic field (Houpt et al., 2003) | 2-deoxy-d-glucose (Watson et al., 1987) | ||
| House Musk Shrew (Suncus murinus) | Cytotoxic drugs: | Cisplatin, cyclophosphamide (Matsuki et al., 1988) | ||
| Intragastric irritants: | Copper sulfate, ipecac (Ueno et al., 1987), LiCl (Parker et al., 2004) | LiCl (Smith et al., 2001a) | ||
| Motion | (Ueno et al., 1988) | (Smith et al., 2001a) | ||
| Radiation | (Torii et al., 1993) | |||
| Nicotine | (Ueno et al., 1987) | Smith et al., 2001a) | ||
| Hormones and neurotransmitters: | Vasopressin (Ikegaya and Matsuki, 2002) | |||
For some stimuli only representative studies or review papers are cited. Only the most common mammalian species used in the study of emesis and malaise are shown. A common set of emetic and nauseogenic stimuli, including cytotoxic drugs, intragastric irritants, etc., are included, but a few less commonly used stimuli are also listed because they demonstrate the broad spectrum of stimuli that can produce emesis, CFA, and pica.
2.2.1. Conditioned flavour avoidance (CFA)
Many species show CFA or learned flavour aversion to foods or flavours associated with toxicosis, including humans (Mattes et al., 1991; Mattes, 1994). CFA is used extensively to study malaise in laboratory animals, particularly the rat. In many cases this effect is simply called a conditioned “taste” aversion or “CTA.” However, there are two problems with this terminology. First, the modality of the conditioned stimulus is not always known, particularly with learned responses to complex foods. The conditioned stimulus could be any one of the three components of flavour, which include gustation, olfaction, or chemaesthesia (touch, temperature, or irritation sensitivity, subserved by trigeminal innervation of the oral cavity), or a combination of these. Therefore the phenomenon is more properly referred to as a conditioned “flavour” avoidance or aversion in a general sense, particularly when the modality of the flavour component for the conditioned stimulus has not been determined.
The second problem is the distinction between conditioned flavour avoidance and aversion. Typically in testing this phenomenon in laboratory animals, a novel flavour is paired with injection of a suspected toxin. When animals are later tested they avoid consumption of a flavoured solution that was associated with toxicosis, demonstrating a CFA. In contrast, a conditioned flavour “aversion” must be tested by measuring consumption of a flavoured fluid that is directly infused through a cannula implanted in the oral cavity (Parker, 1995). This methodological difference can critically affect the interpretation of results because even drugs with rewarding properties, such as amphetamines and cocaine, produce CFA, but do not generate learned flavour aversions in the rat (Parker, 1995); however, there may be important species differences here because some emetic species show amphetamine-induced CFA, e.g., cat and ferret, but Suncus does not (Rabin and Hunt, 1992). Consequently, CFA may not always represent malaise in the rat. For example, an antiemetic drug, a 5-HT3 (5-hydroxytryptamine; serotonin) receptor antagonist, had no effect on CFA but did attenuate learned flavour aversions (tested with intra-oral infusions) produced by intra-peritoneal injections of lithium chloride (LiCl, a gastrointestinal irritant) (Rudd et al., 1998; Limebeer and Parker, 2000). Nearly all of the references cited in the present review refer to results with bottle consumption measures, and are therefore tests of CFA and may not represent learned flavour aversions.
It is likely that learned flavour aversion is an indicator of malaise or nausea, and this type of learning might serve to predict foods that should be avoided. A similar learning process may occur in cancer patients because they often show learned avoidance to foods, or even environmental stimuli, associated with chemotherapy (Mattes et al., 1991; Mattes, 1994; Matteson et al., 2002). In laboratory animals learned flavour aversion produced by emetogenic treatments, including cytotoxic chemotherapy agents, can be blocked by anti-emetic medications. Ondansetron, a 5-HT3 receptor antagonist, and delta-9-tetrahydrocannabinol, a cannabinoid receptor agonist, which inhibit nausea and vomiting in humans (e.g., Cassidy et al., 1988; Massidda and Ionta, 1996), suppress learned flavour aversions in rats produced by intra-peritoneal injections of LiCl or cyclophosphamide, a chemotherapy agent (Limebeer and Parker, 1999, 2000).
The relation between CFA or learned flavour aversion and malaise might be directly assessed by using emetogenic stimuli to produce CFA in emetic species. To date, there are no reports showing conditioned flavour aversion, using intra-oral infusion testing, in an emetic species. However, several studies show toxicosis-induced CFA in emetic species, including human, cat, dog, monkey, Suncus, and ferret (e.g., Jackson and Smith, 1978; Vavilova and Kassil, 1984; Rabin et al., 1986b; Fox et al., 1990; Rabin and Hunt, 1992; Smith et al., 2001a). These studies suggest a complex relation between a toxin’s emetogenic potential and its potency to produce a CFA. For example, although radiation and LiCl produce vomiting in the ferret, LiCl treatment, but not radiation, induced a CFA (Rabin and Hunt, 1992). Further studies using conditioned flavour aversion testing, with intra-oral infusion testing, might clarify the relation between emesis, learned flavour aversion, and CFA.
It is difficult to assess the physiology for the generation of malaise using CFA or learned flavour aversion testing because many pharmacological and lesion treatments to the brain affect neural processes, such as learning and memory, that are not specifically involved in the systems for toxin detection. Additionally, it is very difficult to assess the properties of the toxin detection system using CFA testing because the conditioning protocol involves a delay in testing that is not conducive to a detailed temporal analysis of malaise. For example, it is clear from research on emesis that an analysis of the acute and delayed phases of toxin detection is critical, but this type of investigation is not possible using CFA or learned flavour aversion testing (Hesketh et al., 2003). Although CFA and learned flavour aversion appear to measure the malaise producing potential of a stimulus, this is probably not always true. For example, intra-cerebroventricular infusions of neuropeptide Y produce robust increases in food intake in the rat, but also generate learned flavour aversions (Sipols et al., 1992), which suggests that the hunger stimulus can also produce conditioned flavour aversion.
2.2.2. Kaolin ingestion (pica)
Another way to test for malaise is to measure the consumption of non-nutritive substances following a toxic treatment. Ingestion of dirt or clay, also called pica, in response to toxicosis is a common phenomenon in animals and is also observed in humans (Reid, 1992; Root-Bernstein and Root-Bernstein, 2000; Engel, 2002; Kushner et al., 2004). Rats injected with toxins or subjected to motion consume non-nutritive clay (kaolin) that they would normally not ingest (Mitchell et al., 1977a; Takeda et al., 1993; Santucci et al., 2000; Saeki et al., 2001; Yamamoto et al., 2002a). The presence of pica in mice is controversial with some studies reporting it (Santucci et al., 2000, 2002; Yamamoto et al., 2002a) whilst others do not (Liu et al., 2005). The consumption of clay induced by toxicosis might represent an adaptive response to bind or dilute a toxin in the gastrointestinal tract and reduce its adverse effects on the organism. Clay is particularly effective for binding and diluting toxic chemicals (e.g., Phillips et al., 1995; Sarr et al., 1995; Phillips, 1999), and pica seems to be a specific response in rats because they select clay rather than pebbles or soil when poisoned (Mitchell et al., 1976). However, pica can also be related to nutritional needs; mineral deficiencies in humans and other animals can promote ingestion of clay and other materials that are rich in minerals (e.g., Reynolds et al., 1968; Smith and Halsted, 1970; Roselle, 1970).
Evidence indicates that the positive relation between level of toxicosis and pica is not always maintained at higher doses of a toxin. For example, a high dose of cisplatin, 6 mg/kg, in the rat failed to generate the delayed phase of kaolin consumption, 48–72 h after injection, that was characteristic of a lower dose, 3 mg/kg; and the high dose was associated with successive reductions in food intake and body weight over the 3 days after cisplatin treatment (Rudd et al., 2002). This effect appears to be counterintuitive but high doses of toxins might make animals unable to eat kaolin. However, this phenomenon needs to be investigated using stimuli other than cisplatin as it induces marked gastric stasis and the reduction in kaolin intake may be secondary to this effect.
There are compelling relations between pica, CFA and emesis. Like CFA, pica can be a learned response. Treatment with LiCl or cyclophosphamide produce CFA to a saccharin flavoured solution in the rat and subsequent access to this flavour will stimulate kaolin intake (Mitchell et al., 1977b). Furthermore, there appears to be a close association between the neuropharmacology of toxicosisinduced pica and emesis. Like emesis in cancer patients receiving chemotherapy (Tsukada et al., 2001; Hesketh et al., 2003; de Wit et al., 2004), cisplatin-induced pica in the rat can be characterized into acute and delayed phases that are most sensitive to 5-HT3 and NK1 receptor antagonists, respectively (Saeki et al., 2001; Rudd et al., 2002). However, the relation between emesis and pica is not always so clear. Diphenidol, an anti-emetic drug in humans, is a potent inhibitor of apomorphine-induced pica in the rat (Takeda et al., 1995), but does not affect emesis induced by apomorphine in the dog or ferret (Nakayama et al., 2004). To directly relate pica to emesis it is important to test both phenomena in the same species. Two studies using Suncus have addressed this issue and show that although emesis was induced by treatment with copper sulphate, nicotine, and cisplatin, none of these agents stimulated pica (Yamamoto et al., 2004; Liu et al., 2005).
2.3. Neurohypophyseal hormonal secretions and gastric dysrhythmias as measures of nausea?
Toxins and treatments that produce nausea and vomiting can potentially inhibit behavioural systems that generate CFA and pica, which might lead to an underestimation of the level of malaise. One way to avoid this problem is to measure a correlate of malaise that does not depend on animal behaviour. Blood levels of the neurohypophyseal hormones, vasopressin (ADH) and oxytocin, and gastric dysrhythmia are two physiological parameters that are proposed to be correlates of malaise in animal models and reports of nausea in humans.
Although vasopressin is normally secreted in response to reduced hydration, vasopressin is elevated in well hydrated humans who experience nausea, e.g., nausea produced by gastric distension with a water load can elevate vasopressin levels (Rowe et al., 1979). Many treatments that produce nausea and vomiting, such as apomorphine, chemotherapy, motion, and cholecystokinin, also increase plasma vasopressin to varying extents including to levels exceeding maximal levels for anti-diuresis (Rowe et al., 1979; Fisher et al., 1982; Grant et al., 1986; Feldman et al., 1988; Miaskiewicz et al., 1989; Edwards et al., 1989; Koch et al., 1990b). Plasma vasopressin levels appear to increase slightly before or at the onset of nausea (Miaskiewicz et al., 1989; Koch et al., 1990b). Administration of vasopressin at supraphysiological doses produced nausea in humans, but when vasopressin was infused to match the plasma level during nausea induced by motion nausea was not reported (Kim et al., 1997). Additionally, apomorphine induces nausea in patients with idiopathic diabetes insipidus despite the absence of an increase in plasma vasopressin (Nussey et al., 1988). Although this does not exclude a role for vasopressin in the genesis of nausea in intact individuals, it is a clear that a rise in plasma vasopressin is not essential for the production of nausea under all circumstances and taken together with the studies of vasopressin infusion and plasma levels could indicate that vasopressin requires an additional factor(s) to be present for the induction of nausea when it is released at a lower concentration. This appears to be particularly likely in the case of the circular vection model of motion sickness (Kim et al., 1997).
Animal models also show an increase in neurohypophyseal hormonal secretions after treatments that produce emesis or malaise. Doses of apomorphine and cholecystokinin (CCK) that induce emesis in the ferret also elevate plasma vasopressin (Hawthorn et al., 1988; Billig et al., 2001), and vasopressin is elevated by electrical stimulation of abdominal vagal afferent fibres (Hawthorn et al., 1988). Importantly, it is plasma oxytocin levels, much more than vasopressin, that is elevated following malaise producing treatments in the rat, e.g., LiCl and CCK treatments (Flanagan et al., 1988; Huang et al., 2000). In contrast emetigenic doses of apomorphine and CCK did not produce an increase in plasma oxytocin levels in the ferret (Hawthorn et al., 1988; Billig et al., 2001). These differences in toxicosis induced neurohypophyseal secretions between species needs to be further studied in other emetic and non-emetic species. In the rat, elevated oxytocin has been implicated in the mechanisms by which they cope with stress induced by non-noxious sensory stimuli, whereas noxious stimuli in the rat are associated with corticotrophin releasing factor (CRF) and vasopressin secretion (Uvnas-Moberg, 1998).
Despite a focus on vasopressin secretion as a correlate of nausea (and oxytocin as a correlate of malaise in the rat) other hormones are increased in subjects with nausea induced by apomorphine, ipecac, and motion, including adrenaline, cortisol, growth hormone, prolactin, adrenocorticotrophic hormone, and pancreatic polypeptide (Eversmann et al., 1978; Feldman et al., 1988; Nussey et al., 1988; Page et al., 1990; Xu et al., 1993). It remains to be determined whether any of these hormones are related to the genesis for the sensation of nausea in contrast to being indicators of the generally “stressful” nature of malaise. It would be helpful to have a plasma “endocrine profile” monitoring a wide range of signalling molecules with multiple emetic challenges under carefully controlled conditions to identify consistent directional changes associated with nausea (cf. gene microarray approach).
A second important correlate of nausea is gastric dysrhythmia, which is an abnormal frequency of the myoelectric activity of the stomach. Motion or intravenous infusion of vasopressin elicited gastric dysrhythmia in humans, and the dysrhythmia was recording prior to reported nausea (Stern et al., 1987; Koch et al., 1990a; Caras et al., 1997). Gastric dysrhythmia is closely correlated with reported nausea in a variety of conditions that produce nausea, including surgery, pregnancy and chemotherapy (Pezzolla et al., 1991; Riezzo et al., 1992; DiBaise et al., 2001). Few studies have been conducted on gastric dysrhythmia in animal models of malaise. Gastric dysrhythmia precedes vomiting in the dog induced by electrical stimulation of the vagus (Ueno and Chen, 2004), and intravenous infusion of vasopressin produces gastric dysrhythmia in the dog (Xu et al., 2005).
It is unknown whether gastric dysrhythmia, or neurohypophyseal hormonal release, has any causative role in the genesis of nausea or malaise produced by a broad range of stimuli. Furthermore, although the acute association between gastric dysrhythmia, or neurohypophyseal secretions, and nausea is well established more research needs to focus on whether these physiological parameters are involved in delayed or chronic nausea and malaise. Reports indicate that gastric dysrhythmia is associated with unexplained chronic nausea and vomiting (You et al., 1981; Abell et al., 1988).
3. The role of pain in nausea and vomiting
The perceptions of visceral pain and nausea are largely distinct. Visceral pain is a poorly localized phenomenon that can be very intense. In contrast, nausea is an unsettling feeling or sensation of queasiness in the stomach. Although these phenomena appear to be separate, nausea is often associated with pain, and conversely pain can produce nausea and vomiting. For example, postoperative pain and nausea occur together, and when pain is reduced, nausea is also inhibited (Andersen and Krohg, 1976). It is also not clear to what extent CFA (or learned flavour aversion) or pica in laboratory animals measures responses to pain, generalized stress, or a state of visceral malaise that might relate to the human perception of nausea.
It is possible that nausea and visceral pain share some neural pathways or that one might modulate the other. The spinal nerves that innervate the gut play an important role in producing visceral pain, and when these pathways are electrically stimulated humans report pain (White, 1943), whereas electrical stimulation of the vagal afferent fibres in humans can induce nausea (Lewis, 1942). Assuming that gut viscerosensory pathways are a necessary component for the genesis of nausea, spinal pathways might be involved in the production of nausea because nausea can persist in humans even after sub-diaphragmatic vagotomy (Troncon et al., 1995). In contrast, the integrity of the vagal pathways is necessary for the production of emesis by stimuli acting on the gut (Andrews et al., 1990). An alternative explanation for the persistence of nausea in patients with sub-diaphragmatic vagotomy is that stimulation of cardiac vagal afferent fibres contributes to nausea in these patients. Evidence suggests that stimulation of cardiac vagal afferent fibres can evoke vomiting and nausea (Abrahamsson and Thoren, 1973). Additionally, the nausea in vagotomized patients could have an endocrine origin (possibly via an action on the area postrema) as nausea is associated with an increase in the plasma levels of a number of hormones including adrenaline (see above). It is also clear that pain can enhance nausea, e.g., migraine headache or facial pain increases nausea (Drummond and Granston, 2004, 2005).
4. Neural circuitry for nausea
Much is known about the neural system responsible for emesis. Input systems that activate the vomiting reflex include cerebral, vestibular, area postrema (also known as the chemoreceptor trigger zone, although this name should not be taken to mean that all systemic agents inducing emesis act here), cardiac, and gastrointestinal vagal afferent fibres. These input systems activate a distributed brainstem system, known collectively as the “vomiting centre.” The somatic and autonomic outputs of this system are well established but the circuitry of the vomiting centre is still a focus of intense research. In contrast to the system for emesis relatively little is known about the neural system responsible for nausea. This lack of information is largely due to the difficulty in establishing an animal model of nausea, as well as technical problems in studying this phenomenon in humans using brain imaging techniques.
Although little is known about the neural system for nausea, this system appears to be anatomically distinct from the neural circuits responsible for emesis. It is clear that the vomiting reflex is contained within the brainstem because even decerebrate animals are capable of vomiting and emesis-like behaviour, e.g., the cat (Miller et al., 1994). It is likely that many of the autonomic responses that occur preand post-emesis are responses of the Fvomiting motor program_ or are responses to the Fstress_ of nausea. In fact many of the autonomic signs of motion sickness can be observed in a decorticate person on an airplane (Borison and MacCarthy, 1983), and are unlikely to contribute to the genesis of nausea. Forebrain areas are required for learning a flavour aversion in rat because decerebrate animals fail to show a conditioned response (Grill and Norgren, 1978). However, although specific ablation of forebrain regions block CFA in the rat it is difficult to determine whether these brain regions are important for the experience of malaise, and perhaps nausea in humans, or are involved in memory and learning processes of CFA (for review see Yamamoto et al., 1994). Currently there are no published reports on the effects of brain or peripheral nerve lesions on pica.
Nausea and emesis also appear to be pharmacologically separable. Despite the introduction of 5-HT3 receptor antagonists (e.g. ondansetron, granisetron) for anti-emetic therapy more than half of patients who receive chemotherapy continue to experience nausea (Herrington et al., 2000; Morrow et al., 2000). Even with the newer, tachykinin NK1 receptor antagonists (e.g., aprepitant) there is less than complete control of the nausea component of chemotherapy (Andrews and Rudd, 2004). Nausea is often considered more troublesome for patients because it can be present continuously in contrast to emesis which usually occurs in discrete episodes. It is usually assumed that nausea is a low level stimulus that if increased would result in emesis. However, counter-intuitively, nausea is more difficult to block pharmacologically than emesis (Morrow et al., 2002b), and nausea and vomiting can be dissociated experimentally by adjusting the stimulus intensity. Nausea and vomiting also occur separately in a number of clinical situations; for example, raised intracranial pressure induces emesis but is said to not be preceded by nausea (Lee and Feldman, 1993) and some cancer patients receiving radio-therapy and pregnant women may experience vomiting without nausea (Tierson et al., 1986; Miralbell et al., 1995). In animal studies LiCl and CCK do not always produce emesis even at high doses when malaise is suggested by behavioural (CFA) or physiological measures (elevated plasma vasopressin or oxytocin) (Rabin and Hunt, 1992; Billig et al., 2001; Smith et al., 2001a).
Collectively, these results indicate that nausea and vomiting are generated by neural systems that are at least partially separate. The brainstem is essential for the integration of the emetic signal and coordination of the motor components of emesis, and the projection of information rostrally from the brainstem to “higher” centres is required for the genesis of the sensation for nausea. Identification of the site at which the nausea and emesis signals diverge in the brainstem is essential for targeting therapeutic interventions likely to influence both nausea and vomiting. Whilst a common pathway for nausea and emesis might apply for stimuli acting via the vagal afferent fibres and the area postrema, it is less clear that nausea requires the same neural routing via the brainstem compared to emesis originating from cerebral or vestibular sites. In this case, nausea originating from cerebral or vestibular sites might directly access the “higher” centres involved in the genesis of nausea without engaging the brainstem circuits responsible for emesis (Rudd and Andrews, 2005). It is worth recalling that Dr. Kyrlov, a colleague of Pavlov’s, described studies in the dog showing that nausea, secretion of saliva, vomiting and sleep could all be induced by “the preliminaries of injection” following injection of morphine itself over 5 or 6 days (Pavlov, 1927). In some cases all the symptoms could be induced by “seeing the experimenter”, an observation reminiscent of the reports of nausea and vomiting induced in patients when they have encountered the person who administered chemotherapy outside a hospital setting.
4.1. Visceral afferent fibres
Vagal afferent neurons, connecting the gastrointestinal tract to the brain, play a large role in the generation of vomiting (Andrews et al., 1990), and also serve as a pathway for the induction of nausea. In a biological as opposed to a clinical context, the upper gastrointestinal tract vagal afferents are arguably most important post-ingestive system for the induction of nausea (accompanied by reduced food intake, appetite and delayed gastric emptying) and vomiting if the stimulus is sufficiently intense. These peripheral nerve pathways for the initiation of vomiting have been most thoroughly investigated using cancer chemotherapy agents (for review see Rudd and Andrews, 2005). Many chemotherapy agents, such as cisplatin, cause the release of 5-HT from entero-endocrine cells (enterochromaffin) of the gastrointestinal tract that leads to the activation of vagal afferent fibres containing 5-HT3 receptors (Andrews et al., 1988; Minami et al., 1997; Hillsley and Grundy, 1999). 5-HT3 receptor antagonists are very effective blockers of the acute phase of vomiting and partially control the nausea produced by chemotherapy (e.g., Cassidy et al., 1988; Massidda and Ionta, 1996; Morrow et al., 2002b). However, despite the introduction of 5-HT3 receptor antagonists for anti-emetic therapy more than half of patients who receive chemotherapy continue to experience nausea (Herrington et al., 2000; Morrow et al., 2000). Unlike emesis, 5-HT3 receptor antagonists might have their primary effect on nausea through direct action on the brain. These antagonist compounds readily cross the blood brain barrier where they could act on 5-HT3 receptors located throughout the neuroaxis, although present in particularly high levels in the NTS.
Abdominal vagal afferent fibres are not essential for the sensation of nausea because even humans with bilateral abdominal vagotomy experience nausea, which might suggest that spinal pathways play a role in the genesis of nausea by stimuli acting on the gut. There is little information on the role of visceral nerve pathways on animal studies of CFA or pica. Early reports showed that abdominal vagotomy did not affect LiCl-induced CFA (Martin et al., 1978), but did attenuate intra-gastric copper sulphate and motion-induced CFA in the rat (Coil et al., 1978; Fox and McKenna, 1988). There is no information on the effects of vagal lesions on the acquisition of conditioned flavour aversion, using intra-oral infusion testing. Furthermore, as mentioned above, there are no reports on the effects of vagal lesions on toxicosis-induced pica.
4.2. Direct action of stimuli on the brain?
It is also possible that toxins and other treatments act on the brain directly to produce nausea. Activation of the area postrema has been suggested to play a role in CFA produced by copper sulphate and other blood borne toxins (Coil and Norgren, 1981; Borison et al., 1984; Rabin et al., 1985). However, evidence to support this hypothesis has relied on AP ablation experiments, which, as noted above, are difficult to interpret. These studies also did not directly measure learned flavour aversion using the intra-oral infusion method.
Recent research showed that nausea associated with chemotherapy is correlated with a reduction of plasma cortisol levels in cancer patients, which indicates an effect (direct or indirect) on the hypothalamic-pituitary-adrenal axis (Hursti et al., 1993; Morrow et al., 2002a). Studies in rat show elevated plasma cortisol levels with the production of CFA (Smotherman, 1985). Additionally (as mentioned above), there are many potential candidate hormones that might contribute to nausea, including vasopressin, oxytocin, adrenaline, growth hormone, prolactin, adrenocorticotrophic hormone, and pancreatic polypeptide (Eversmann et al., 1978; Feldman et al., 1988; Nussey et al., 1988; Page et al., 1990; Xu et al., 1993). However, the challenge with all the systemic signals for nausea is to understand where and how they gain access to the central nervous system (e.g. via circumventricular organs) and how they then induce the sensation of nausea. It is also possible that some of these systemic agents act either on the gut to disrupt function and it is this which is signalled via the visceral afferents or that they act directly on receptors on the visceral afferent terminals.
4.3. The stimulus and coding of signals for nausea and vomiting
Despite the well established role of the abdominal vagal afferents in emesis, and to a much lesser extent nausea, we have no clear understanding of the information code that is conveyed to the brain by the visceral afferent fibres and how this is encoded to signal nausea or induce vomiting. Activation of the vagal afferent fibres by toxins in the gut appears to operate via detection of toxins by enteroendocrine cells that then release 5-HT as a paracrine factor that activates 5-HT3 receptors on vagal afferent fibres. 5-HT seems to be the predominant neurotransmitter in the abdominal vagal system (Uneyama et al., 2002; Horn et al., 2004) although enteroendocrine cells such as the enterochromaffin cells which contain high levels of 5-HT also contain substance P. There is neurophysiological evidence for complex interactions between 5-HT and SP and the activation of vagal afferent fibres via 5-HT3 and NK1 receptors (Minami et al., 2001). Evidence suggests that serotonin signalling is also used for the detection of nutrients, such as carbohydrates (Li et al., 2001; Zhu et al., 2001), which might serve to promote nutrient absorption and processing and modify food intake. It is therefore puzzling how the vagal system can accomplish these multiple functions, including the sensing of toxins, using a sensory system that appears to be highly likely to provide ambiguous information to the brain.
5. Conclusions
It is clear from the current review that investigating the physiology of nausea and vomiting is very challenging, and much of this difficulty stems from limitations that exist when studying animal models. It is not known which species is most predictive of the neuropharmacology of emesis in humans when all types of emetic stimuli are considered, although in the case of chemotherapy-induced emesis the ferret is probably the species used first to test a candidate antiemetic agent. It is particularly difficult to decide what animal model is most appropriate for studying the symptom of nausea. CFA and pica are potential model responses, usually studied in species lacking an emetic reflex, that can be used to address this issue, however, there are likely to be significant overlaps between systems that generate emesis, CFA, and pica (or associated measures of plasma hormones and gastric dysrythmia) with no guarantee that any of these responses provide insights into the physiology of human nausea. It is therefore important that results from animal experiments concerning nausea be validated in human studies and that the results of such studies are in turn used to refine appropriate models and abandon inappropriate ones. Ideally we should work towards one widely accepted model of nausea and vomiting for pre-clinical testing of candidate therapies whilst simultaneously increasing human studies particularly in the area of nausea.
The current understanding of nausea and vomiting is also limited by other factors that might be independent of species selection. It is not clear how pain and stress are related to nausea and vomiting. There are a large number of autonomic efferent and hormonal responses that accompany vomiting and the experience of nausea but many of these effects appear to be general Fstress_ responses. It also remains an important question as to how the vagal afferent system codes information related to the genesis of nausea and vomiting. These unresolved issues guarantee that the study of nausea and vomiting will remain an active area of research. This is particularly true because an understanding of the physiology and pharmacology of nausea and vomiting is of cardinal importance for developing Fclean_ drugs that target clinically important diseases as many potential therapeutic approaches have nausea and vomiting as dose limiting toxicities and also for developing drugs acting to reduce both nausea and vomiting in a diverse range of clinical settings.
Acknowledgements
The authors are supported by grants from the National Institutes of Health (DK065971) and Glaxo Smith Kline.
References
- Abell TL, Kim CH, Malagelada JR. Idiopathic cyclic nausea and vomiting—a disorder of gastrointestinal motility? Mayo Clin. Proc. 1988;63:1169–1175. doi: 10.1016/s0025-6196(12)65401-9. [DOI] [PubMed] [Google Scholar]
- Abrahamsson H, Thoren P. Vomiting and reflex vagal relaxation of the stomach elicited from heart receptors in the cat. Acta Physiol. Scand. 1973;88:433–439. doi: 10.1111/j.1748-1716.1973.tb05472.x. [DOI] [PubMed] [Google Scholar]
- Ader R. Conditioned adrenocortical steroid elevations in the rat. J. Comp. Physiol. Psychol. 1976;90:1156–1163. doi: 10.1037/h0077290. [DOI] [PubMed] [Google Scholar]
- Amber EI, Henderson RA, Adeyanju JB, Gyang EO. Singledrug chemotherapy of canine transmissible venereal tumor with cyclophosphamide, methotrexate, or vincristine. J. Vet. Intern. Med. 1990;4:144–147. doi: 10.1111/j.1939-1676.1990.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Andersen R, Krohg K. Pain as a major cause of postoperative nausea. Can. Anaesth. Soc. J. 1976;23:366–369. doi: 10.1007/BF03005916. [DOI] [PubMed] [Google Scholar]
- Andrew BL. The nervous control of the cervical oesophagus of the rat during swallowing. J. Physiol. 1956;134:729–740. doi: 10.1113/jphysiol.1956.sp005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PLR. Why do some animals lack a vomiting reflex? Physiol. Zool. 1995;68:61. [Google Scholar]
- Andrews PLR, Rudd JA. The role of Tachykinins and the Tachykinin NK1 receptor in nausea and emesis. In: Holzer P, editor. Tachykinins, Springer: Handbook of Experimental Pharmacology; 2004. pp. 359–440. [Google Scholar]
- Andrews PLR, Rapeport WG, Sanger GJ. Neuropharmacology of emesis induced by anti-cancer therapy. Trends Pharmacol. Sci. 1988;9:334–341. doi: 10.1016/0165-6147(88)90106-x. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can. J. Physiol. Pharm. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Axelsson M, Franklin C, Holmgren S. The emetic reflex in a reptile (Crocodylus porosus) J. Exp. Biol. 2000;203:1625–1632. doi: 10.1242/jeb.203.10.1625. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Kovacs M, Watson JW. The anti-emetic action of the neurokinin(1) receptor antagonist CP-99,994 does not require the presence of the area postrema in the dog. Neurosci. Lett. 2001;314:102–104. doi: 10.1016/s0304-3940(01)02269-8. [DOI] [PubMed] [Google Scholar]
- Andrews PLR, Hoyle CHV, Ngoka T, Smith GE. Differences in the vagal innervation of the oesophagus may explain the lack of emesis in rodents. Neurogastroenterol. Motil. 2003;15:25. [Google Scholar]
- Arwas S, Rolnick A, Lubow RE. Conditioned taste aversion in humans using motion-induced sickness as the US. Behav. Res. Ther. 1989;27:295–301. doi: 10.1016/0005-7967(89)90049-1. [DOI] [PubMed] [Google Scholar]
- Aung HH, Mehendale SR, Xie JT, Moss J, Yuan CS. Methylnaltrexone prevents morphine-induced kaolin intake in the rat. Life Sci. 2004;74:2685–2691. doi: 10.1016/j.lfs.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Bailey PL, Rhondeau S, Schafer PG, Lu JK, Timmins BS, Foster W, Pace NL, Stanley TH. Dose–response pharmacology of intrathecal morphine in human volunteers. Anesthesiology. 1993;79:49–59. doi: 10.1097/00000542-199307000-00010. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Bunce KT, Naylor RJ, Rudd JA. The actions of fentanyl to inhibit drug-induced emesis. Neuropharmacology. 1991;30:1073–1083. doi: 10.1016/0028-3908(91)90136-y. [DOI] [PubMed] [Google Scholar]
- Bayley TM, Dye L, Jones S, DeBono M, Hill AJ. Food cravings and aversions during pregnancy: relationships with nausea and vomiting. Appetite. 2002;38:45–51. doi: 10.1006/appe.2002.0470. [DOI] [PubMed] [Google Scholar]
- Beleslin DB, Krstic SK, Stefanovic-Denic K, Strbac M, Micic D. Inhibition by morphine and morphine-like drugs of nicotineinduced emesis in cats. Brain Res. Bull. 1981;6:451–453. doi: 10.1016/s0361-9230(81)80017-2. [DOI] [PubMed] [Google Scholar]
- Bergman J, Glowa JR. Suppression of behaviour by food pelletlithium chloride pairings in squirrel monkeys. Pharmacol. Biochem. Behav. 1986;25:973–978. doi: 10.1016/0091-3057(86)90072-9. [DOI] [PubMed] [Google Scholar]
- Bielavska E, Kren V, Musilova A, Zidek V, Pravenec M. Genome scanning of the HXB/BXH sets of recombinant inbred strains of the rat for quantitative trait loci associated with conditioned taste aversion. Behav. Genet. 2002;32:51–56. doi: 10.1023/a:1014407928865. [DOI] [PubMed] [Google Scholar]
- Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecystokinin. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2001;281:R1243–R1255. doi: 10.1152/ajpregu.2001.281.4.R1243. [DOI] [PubMed] [Google Scholar]
- Borison HL, MacCarthy LE. Neuropharmacologic mechanisms of emesis. In: Laszlo J, editor. Antiemetics and Cancer Chemotherapy. Baltimore: Williams and Wilkins; 1983. pp. 6–20. [Google Scholar]
- Borison HL, Borison R, McCarthy LE. Role of the area postrema in vomiting and related functions. Fed. Proc. 1984;43:2955–2958. [PubMed] [Google Scholar]
- Braveman NS. Poison-based avoidance learning with flavoured or colored water in guinea pigs. Learn. Motiv. 1974;5:182–194. [Google Scholar]
- Brizzee KR. Effect of localized brain stem lesions and supradiaphragmatic vagotomy on X-irradiation emesis in the monkey. Am. J. Physiol. 1956;187:567–570. doi: 10.1152/ajplegacy.1956.187.3.567. [DOI] [PubMed] [Google Scholar]
- Brizzee KR, Neal LM, Williams PM. The chemoreceptor trigger zone for emesis in the monkey. Am. J. Physiol. 1955;180:659–662. doi: 10.1152/ajplegacy.1955.180.3.659. [DOI] [PubMed] [Google Scholar]
- Caras SD, Soykan I, Beverly V, Lin Z, McCallum RW. The effect of intravenous vasopressin on gastric myoelectrical activity in human subjects. Neurogastroenterol. Motil. 1997;9:151–156. doi: 10.1046/j.1365-2982.1997.d01-37.x. [DOI] [PubMed] [Google Scholar]
- Carr DH, Scott PC, Titchen DA. Manometric and electromyographic observations of the oesophagus of sheep in eructation, regurgitation and swallowing. Q. J. Exp. Physiol. 1983;68:661–674. doi: 10.1113/expphysiol.1983.sp002756. [DOI] [PubMed] [Google Scholar]
- Carrell LE, Cannon DS, Best MR, Stone MJ. Nausea and radiation-induced taste aversions in cancer patients. Appetite. 1986;7:203–208. doi: 10.1016/s0195-6663(86)80025-3. [DOI] [PubMed] [Google Scholar]
- Cassidy J, Raina V, Lewis C, Adams L, Soukop M, Rapeport WG, Zussman BD, Rankin EM, Kaye SB. Pharmacokinetics and anti-emetic efficacy of BRL 43694, a new selective 5-HT3 antagonist. Br. J. Cancer. 1988;58:651–653. doi: 10.1038/bjc.1988.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian MS, York RG, Hoberman AM, Diener RM, Fisher LC, Gates GA. Biodisposition of dibromoacetic acid (DBA) and bromodichloromethane (BDCM) administered to rats and rabbits in drinking water during range-finding reproduction and developmental toxicity studies. Int. J. Toxicol. 2001;20:239–253. doi: 10.1080/109158101750408064. [DOI] [PubMed] [Google Scholar]
- Coil JD, Norgren R. Taste aversions conditioned with intravenous copper sulphate: attenuation by ablation of the area postrema. Brain Res. 1981;212:425–433. doi: 10.1016/0006-8993(81)90474-1. [DOI] [PubMed] [Google Scholar]
- Coil JD, Hankins WG, Jenden DJ, Garcia J. The attenuation of a specific cue-to-consequence association by antiemetic agents. Psychopharmacology (Berlin) 1978;56:21–25. doi: 10.1007/BF00571403. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Mattsson JL. Control of radiation-induced emesis with promethazine, cimetidine, thiethylperazine, or naloxone. Am. J. Vet. Res. 1979;40:1057–1061. [PubMed] [Google Scholar]
- Corbett RW, Ryan C, Weinrich SP. Pica in pregnancy: does it affect pregnancy outcomes? MCN. Am. J. Matern. Child Nursing. 2003;28:183–189. doi: 10.1097/00005721-200305000-00009. [DOI] [PubMed] [Google Scholar]
- Corcoran ML, Fox RA, Daunton NG. The susceptibility of rhesus monkeys to motion sickness. Aviat. Space Environ. Med. 1990;61:807–809. [PubMed] [Google Scholar]
- Cordts RE, Yochmowitz MG, Hardy KA. Evaluation of domperidone as a modifier of gamma-radiation-induced emesis. Int. J. Radiat. Oncol. Biol. Phys. 1987;13:1333–1337. doi: 10.1016/0360-3016(87)90225-2. [DOI] [PubMed] [Google Scholar]
- Costall B, Domeney AM, Naylor RJ. A model of nausea and emesis in the common marmoset. Br. J. Pharmacol. 1987;88:375. [Google Scholar]
- Costello DJ, Borison HL. Naloxone antagonizes narcotic self blockade of emesis in the cat. J. Pharmacol. Exp. Ther. 1977;203:222–230. [PubMed] [Google Scholar]
- Crampton GH, Daunton NG. Systemic naloxone increases the incidence of motion sickness in the cat. Pharmacol. Biochem. Behav. 1983;19:827–829. doi: 10.1016/0091-3057(83)90088-6. [DOI] [PubMed] [Google Scholar]
- Darolova A. Food composition in the eagle-owl (Bubo bubo L., 1758) in Small Carpathians (Czechoslovakia) Biologia. 1991;45:831–840. [Google Scholar]
- Davis CJ, Harding RK, Leslie RA, Andrews PLR. The organisation of vomiting as a protective reflex. In: Davis CJ, Lake- Bakaar GV, Grahame-Smith DG, editors. Nausea and Vomiting, Mechanisms and Treatment. Berlin: Springer-Verlag; 1986. pp. 65–75. [Google Scholar]
- Dawkins R. The Ancestor’s Tale, Rodents and Rabbitkind. London, UK: Weidenfield & Nicholson; 2004. pp. 152–161. [Google Scholar]
- de Wit R, Herrstedt J, Rapoport B, Carides AD, Guoguang-Ma J, Elmer M, Schmidt C, Evans JK, Horgan KJ. The oral NK(1) antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy a combined analysis of two randomised, placebo-controlled phase III clinical trials. Eur. J. Cancer. 2004;40:403–410. [PubMed] [Google Scholar]
- DiBaise JK, Brand RE, Lyden E, Tarantolo SR, Quigley EM. Gastric myoelectrical activity and its relationship to the development of nausea and vomiting after intensive chemotherapy and autologous stem cell transplantation. Am. J. Gastroenterol. 2001;96:2873–2881. doi: 10.1111/j.1572-0241.2001.04241.x. [DOI] [PubMed] [Google Scholar]
- Dibattista D. Conditioned taste aversion produced by 2-deoxy-dglucose in rats and hamsters. Physiol. Behav. 1988;44:189–192. doi: 10.1016/0031-9384(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J. Comp. Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Drummond PD, Granston A. Facial pain increases nausea and headache during motion sickness in migraine sufferers. Brain. 2004;127:526–534. doi: 10.1093/brain/awh061. [DOI] [PubMed] [Google Scholar]
- Drummond PD, Granston A. Painful stimulation of the temple induces nausea, headache and extracranial vasodilation in migraine sufferers. Cephalalgia. 2005;25:16–22. doi: 10.1111/j.1468-2982.2004.00810.x. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Carmichael J, Baylis PH, Harris AL. Arginine vasopressin—a mediator of chemotherapy induced emesis? Br. J. Cancer. 1989;59:467–470. doi: 10.1038/bjc.1989.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel C. Wild Health: How Animals Keep Themselves Well and What We Can Learn From Them. New York: Houghton Mifflin; 2002. [Google Scholar]
- Ervin GN, Mosher JT, Birkemo LS, Johnson MF. Multiple, small doses of cholecystokinin octapeptide are more efficacious at inducing taste aversion conditioning than single, large doses. Peptides. 1995;16:539–545. doi: 10.1016/0196-9781(95)00001-z. [DOI] [PubMed] [Google Scholar]
- Etscorn F. Sucrose aversions in mice as a result of injected nicotine or passive tobacco smoke inhalation. Bull. Psychon. Soc. 1980;15:54–56. [Google Scholar]
- Etscorn F, Moore GA, Hagen LS, Caton TM, Sanders DL. Saccharin aversions in hamsters as a result of nicotine injections. Pharmacol. Biochem. Behav. 1986;24:567–570. doi: 10.1016/0091-3057(86)90559-9. [DOI] [PubMed] [Google Scholar]
- Etscorn F, Moore GA, Scott EP, Hagen LS, Caton TM, Sanders DL, Divine KK. Conditioned saccharin aversions in rats as a result of cutaneous nicotine or intraperitoneal nicotine administered in divided doses. Pharmacol. Biochem. Behav. 1987;28:495–502. doi: 10.1016/0091-3057(87)90512-0. [DOI] [PubMed] [Google Scholar]
- Eversmann T, Gottsmann M, Uhlich E, Ulbrecht G, von WK, Scriba PC. Increased secretion of growth hormone, prolactin, antidiuretic hormone, and cortisol induced by the stress of motion sickness. Aviat. Space Environ. Res. 1978;49:53–57. [PubMed] [Google Scholar]
- Feldman M, Samson WK, O’Dorisio TM. Apomorphineinduced nausea in humans: release of vasopressin and pancreatic polypeptide. Gastroenterology. 1988;95:721–726. doi: 10.1016/s0016-5085(88)80020-9. [DOI] [PubMed] [Google Scholar]
- Fetting JH, McCarthy LE, Borison HL, Colvin M. Vomiting induced by cyclophosphamide and phosphoramide mustard in cats. Cancer Treat. Rep. 1982;66:1625–1629. [PubMed] [Google Scholar]
- Fisher RD, Rentschler RE, Nelson JC, Godfrey TE, Wilbur DW. Elevation of plasma antidiuretic hormones (ADH) associated with chemotherapy-induced emesis in man. Cancer Treat. Rep. 1982;66:25–29. [PubMed] [Google Scholar]
- Flanagan LM, Verbalis JG, Stricker EM. Naloxone potentiation of effects of cholecystokinin and lithium chloride on oxytocin secretion, gastric motility and feeding. Neuroendocrinology. 1988;48:668–673. doi: 10.1159/000125080. [DOI] [PubMed] [Google Scholar]
- Fox RA. Poison aversion and sexual behaviour in the golden hamster. Psychol. Rep. 1977;41:993–994. doi: 10.2466/pr0.1977.41.3.993. [DOI] [PubMed] [Google Scholar]
- Fox RA, McKenna S. Conditioned taste aversion induced by motion is prevented by selective vagotomy in the rat. Behav. Neural Biol. 1988;50:275–284. doi: 10.1016/s0163-1047(88)90954-5. [DOI] [PubMed] [Google Scholar]
- Fox RA, Corcoran M, Brizzee KR. Conditioned taste aversion and motion sickness in cats and squirrel monkeys. Can. J. Physiol. Pharm. 1990;68:269–278. doi: 10.1139/y90-041. [DOI] [PubMed] [Google Scholar]
- Fukui H, Yamamoto M, Sasaki S, Sato S. Involvement of 5-HT3 receptors and vagal afferents in copper sulphate-and cisplatin-induced emesis in monkeys. Eur. J. Pharmacol. 1993;249:13–18. doi: 10.1016/0014-2999(93)90656-3. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Yamada K. The alpha-naphthoxyacetic acid-elicited retching involves dopaminergic inhibition in mice. Pharmacol. Biochem. Behav. 1980;12:735–738. doi: 10.1016/0091-3057(80)90158-6. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Conditoned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Gardner CJ, Armour DR, Beattie DT, Gale JD, Hawcock AB, Kilpatrick GJ, Twissell DJ, Ward P. GR205171: a novel antagonist with high affinity for the tachykinin NK1 receptor, and potent broad-spectrum anti-emetic activity. Regul. Pept. 1996;65:45–53. doi: 10.1016/0167-0115(96)00071-7. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Grant PJ, Hughes JR, Dean HG, Davies JA, Prentice CR. Vasopressin and catecholamine secretion during apomorphine-induced nausea mediate acute changes in haemostatic function in man. Clin. Sci. (Lond.) 1986;71:621–624. doi: 10.1042/cs0710621. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- Gylys JA, Doran KM, Buyniski JP. Antagonism of cisplatin induced emesis in the dog. Res. Commun. Chem. Pathol. Pharmacol. 1979;23:61–68. [PubMed] [Google Scholar]
- Harrison WA, Lipe WA, Decker WJ. Apomorphine-induced emesis in the dog: comparison of routes of administration. J. Am. Vet. Med. Assoc. 1972;160:85–86. [PubMed] [Google Scholar]
- Hasegawa S, Takeda N, Morita M, Horii A, Koizuka I, Kubo T, Matsunaga T. Vestibular, central and gastral triggering of emesis. A study on individual susceptibility in rats. Acta Oto-Laryngol. 1992;112:927–931. doi: 10.3109/00016489209137492. [DOI] [PubMed] [Google Scholar]
- Hawthorn J, Andrews PL, Ang VT, Jenkins JS. Differential release of vasopressin and oxytocin in response to abdominal vagal afferent stimulation or apomorphine in the ferret. Brain Res. 1988;438:193–198. doi: 10.1016/0006-8993(88)91338-8. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Kwan P, Young RR, Lagow E, Lagrone L, Riggs MW. Randomized, multicenter comparison of oral granisetron and oral ondansetron for emetogenic chemotherapy. Pharmacotherapy. 2000;20:1318–1323. doi: 10.1592/phco.20.17.1318.34894. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Van BS, Aapro M, Tattersall FD, Naylor RJ, Hargreaves R, Carides AD, Evans JK, Horgan KJ. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur. J. Cancer. 2003;39:1074–1080. doi: 10.1016/s0959-8049(02)00674-3. [DOI] [PubMed] [Google Scholar]
- Hikami K, Hasegawa Y, Matsuzawa T. Social transmission of food preferences in Japanese monkeys (Macaca fuscata) after mere exposure or aversion training. J. Comp. Psychol. 1990;104:233–237. doi: 10.1037/0735-7036.104.3.233. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Grundy D. Plasticity in the mesenteric afferent response to cisplatin following vagotomy in the rat. J. Auton. Nerv. Syst. 1999;76:93–98. doi: 10.1016/s0165-1838(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Hobbs SH, Clingerman H, Elkins RL. Illness-induced taste aversions in normal and bulbectomized hamsters. Physiol. Behav. 1976;17:235–238. doi: 10.1016/0031-9384(76)90070-6. [DOI] [PubMed] [Google Scholar]
- Holloway RH. Systemic pharmacomodulation of transient lower esophageal sphincter relaxations. Am. J. Med. 2001;111:178S–185S. doi: 10.1016/s0002-9343(01)00853-1. [DOI] [PubMed] [Google Scholar]
- Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibres in the rat by the anti-cancer agent cisplatin. Auton. Neurosci. 2004;115:74–81. doi: 10.1016/j.autneu.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Pittman DW, Barranco JM, Brooks EH, Smith JC. Behavioural effects of high-strength static magnetic fields on rats. J. Neurosci. 2003;23:1498–1505. doi: 10.1523/JNEUROSCI.23-04-01498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Sved AF, Stricker EM. Vasopressin and oxytocin release evoked by NaCl loads are selectively blunted by area postrema lesions. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2000;278:R732–R740. doi: 10.1152/ajpregu.2000.278.3.R732. [DOI] [PubMed] [Google Scholar]
- Hursti TJ, Fredrikson M, Steineck G, Borjeson S, Furst CJ, Peterson C. Endogenous cortisol exerts antiemetic effect similar to that of exogenous corticosteroids. Br. J. Cancer. 1993;68:112–114. doi: 10.1038/bjc.1993.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison SL., Jr Taste aversion in albino rats using centrifugal spin as an unconditioned stimulus. Psychol. Rep. 1973;33:467–470. doi: 10.2466/pr0.1973.33.2.467. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Matsuki N. Vasopressin induces emesis in Suncus murinus. Jpn. J. Pharmacol. 2002;89:324–326. doi: 10.1254/jjp.89.324. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Smith JW. A comparison of two aversion treatment methods for alcoholism. J. Stud. Alcohol. 1978;39:187–191. doi: 10.15288/jsa.1978.39.187. [DOI] [PubMed] [Google Scholar]
- Kayashima N, Hayama T. Reproducibility of copper sulphate emesis by oral administration in dogs. Nippon Yakurigaku Zasshi. 1975;71:169–173. doi: 10.1254/fpj.71.169. [DOI] [PubMed] [Google Scholar]
- Kayashima N, Hyama T. Reproducibility of emesis by orally administrated copper sulphate in cats. Nippon Yakurigaku Zasshi. 1976;72:287–291. [PubMed] [Google Scholar]
- Kim MS, Chey WD, Owyang C, Hasler WL. Role of plasma vasopressin as a mediator of nausea and gastric slow wave dysrhythmias in motion sickness. Am. J. Physiol. 1997;272:G853–G862. doi: 10.1152/ajpgi.1997.272.4.G853. [DOI] [PubMed] [Google Scholar]
- Kimeldorf DJ, Garcia J, Rubadeau DO. Radiation-induced conditioned avoidance behaviour in rats, mice, and cats. Radiat. Res. 1960;12:710–718. [PubMed] [Google Scholar]
- King GL. Animal models in the study of vomiting. Can. J. Physiol. Pharm. 1990;68:260–268. doi: 10.1139/y90-040. [DOI] [PubMed] [Google Scholar]
- Kinney NE, Wright JW, Harding JW. Motion-induced aversions during and after recovery from olfactory nerve section in mice. Physiol. Behav. 1993;53:631–633. doi: 10.1016/0031-9384(93)90166-d. [DOI] [PubMed] [Google Scholar]
- Koch KL, Stern RM, Vasey MW, Seaton JF, Demers LM, Harrison TS. Neuroendocrine and gastric myoelectrical responses to illusory self-motion in humans. Am. J. Physiol. 1990a;258:E304–E310. doi: 10.1152/ajpendo.1990.258.2.E304. [DOI] [PubMed] [Google Scholar]
- Koch KL, Summy-Long J, Bingaman S, Sperry N, Stern RM. Vasopressin and oxytocin responses to illusory self-motion and nausea in man. J. Clin. Endocrinol. Metab. 1990b;71:1269–1275. doi: 10.1210/jcem-71-5-1269. [DOI] [PubMed] [Google Scholar]
- Krane RV, Sinnamon HM, Thomas GJ. Conditioned taste aversions and neophobia in rats with hippocampal lesions. Comp. Physiol. Psychol. 1976;90:680–693. doi: 10.1037/h0077236. [DOI] [PubMed] [Google Scholar]
- Kushner RF, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J. Am. Diet. Assoc. 2004;104:1393–1397. doi: 10.1016/j.jada.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Lee M, Feldman M. Nausea and vomiting. In: Sleisenger MH, Fordtran JS, editors. Gastrointestinal Diseas. Philadelphia, USA: W.B. Saunders Co.; 1993. pp. 509–523. [Google Scholar]
- Lefebvre RA, Willems JL, Bogaert MG. Gastric relaxation and vomiting by apomorphine, morphine and fentanyl in the conscious dog. Eur. J. Pharmacol. 1981;69:139–145. doi: 10.1016/0014-2999(81)90408-8. [DOI] [PubMed] [Google Scholar]
- Levine AS, Sievert CE, Morley JE, Gosnell BA, Silvis SE. Peptidergic regulation of feeding in the dog (Canis familiaris) Peptides. 1984;5:675–679. doi: 10.1016/0196-9781(84)90005-6. [DOI] [PubMed] [Google Scholar]
- Lewis T. Pain. New York, USA: Macmillan; 1942. p. 192. [Google Scholar]
- Li BU, Fleisher DR. Cyclic vomiting syndrome: features to be explained by a pathophysiologic model. Dig. Dis. Sci. 1999;44:13S–18S. [PubMed] [Google Scholar]
- Li Y, Wu XY, Zhu JX, Owyang C. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am. J. Physiol.: Gastrointest. Liver Physiol. 2001;281:G916–G923. doi: 10.1152/ajpgi.2001.281.4.G916. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. Delta-9-tetrahydrocannabinol interferes with the establishment and the expression of conditioned rejection reactions produced by cyclophosphamide: a rat model of nausea. NeuroReport. 1999;10:3769–3772. doi: 10.1097/00001756-199912160-00009. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. J. Exp. Psychol., Anim. Behav. Processes. 2000;26:371–384. doi: 10.1037//0097-7403.26.4.371. [DOI] [PubMed] [Google Scholar]
- Liu J, Kashimura S, Hara K, Zhang G. Death following cupric sulphate emesis. J. Toxicol., Clin. Toxicol. 2001;39:161–163. doi: 10.1081/clt-100103832. [DOI] [PubMed] [Google Scholar]
- Liu Y-L, Malik N, Sanger GJ, Friedman MI, Andrews PLR. Pica—A model of nausea? Species differences in response to cisplatin. Physiol. Behav. 2005;85:271–277. doi: 10.1016/j.physbeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Lockwood DR, Kwon B, Smith JC, Houpt TA. Behavioural effects of static high magnetic fields on unrestrained and restrained mice. Physiol. Behav. 2003;78:635–640. doi: 10.1016/s0031-9384(03)00040-4. [DOI] [PubMed] [Google Scholar]
- Lopez LB, Ortega Soler CR, de Portela ML. Pica during pregnancy: a frequently underestimated problem. Arch. Latinoam. Nutr. 2004;54:17–24. [PubMed] [Google Scholar]
- Martin JR, Cheng FY, Novin D. Acquisition of learned taste aversion following bilateral subdiaphragmatic vagotomy in rats. Physiol. Behav. 1978;21:13–17. doi: 10.1016/0031-9384(78)90269-x. [DOI] [PubMed] [Google Scholar]
- Massidda B, Ionta MT. Prevention of delayed emesis by a single intravenous bolus dose of 5-HT3-receptor-antagonist in moderately emetogenic chemotherapy. J. Chemother. 1996;8:237–242. doi: 10.1179/joc.1996.8.3.237. [DOI] [PubMed] [Google Scholar]
- Matsuki N, Ueno S, Kaji T, Ishihara A, Wang CH, Saito H. Emesis induced by cancer chemotherapeutic agents in the Suncus murinus: a new experimental model. Jpn. J. Pharmacol. 1988;48:303–306. doi: 10.1254/jjp.48.303. [DOI] [PubMed] [Google Scholar]
- Mattes RD. Prevention of food aversions in cancer patients during treatment. Nutr. Cancer. 1994;21:13–24. doi: 10.1080/01635589409514300. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Curran WJ, Jr, Powlis W, Whittington R. A descriptive study of learned food aversions in radiotherapy patients. Physiol. Behav. 1991;50:1103–1109. doi: 10.1016/0031-9384(91)90568-9. [DOI] [PubMed] [Google Scholar]
- Matteson S, Roscoe J, Hickok J, Morrow GR. The role of behavioural conditioning in the development of nausea. J. Obstet. Gynecol. 2002;186:S239. doi: 10.1067/mob.2002.122597. [DOI] [PubMed] [Google Scholar]
- McCarthy LE, Borison HL. Cisplatin-induced vomiting eliminated by ablation of the area postrema in cats. Cancer Treat. Rep. 1984;68:401–404. [PubMed] [Google Scholar]
- McCutcheon B, Ballard M, McCaffrey RJ. Intraperitoneally injected cholecystokinin-octapeptide activates pica in rats. Physiol. Behav. 1992;51:543–547. doi: 10.1016/0031-9384(92)90177-4. [DOI] [PubMed] [Google Scholar]
- Miaskiewicz SL, Stricker EM, Verbalis JG. Neurohypophyseal secretion in response to cholecystokinin but not meal-induced gastric distention in humans. J. Clin. Endocrinol. Metab. 1989;68:837–843. doi: 10.1210/jcem-68-4-837. [DOI] [PubMed] [Google Scholar]
- Miller AD, Nonaka S, Jakus J. Brain areas essential or nonessential for emesis. Brain Res. 1994;647:255–264. doi: 10.1016/0006-8993(94)91325-0. [DOI] [PubMed] [Google Scholar]
- Minami M, Ogawa T, Endo T, Hamaue N, Hirafuji M, Yoshioka M, Blower PR, Andrews PL. Cyclophosphamide increases 5-hydroxytryptamine release from the isolated ileum of the rat. Res. Commun. Mol. Pathol. Pharmacol. 1997;97:13–24. [PubMed] [Google Scholar]
- Minami M, et al. Effects of CP-99, 994, a tachykinin NK(1) receptor antagonist, on abdominal afferent vagal activity in ferrets: evidence for involvement of NK(1) and 5-HT(3) receptors. Eur. J. Pharmacol. 2001;428:215–220. doi: 10.1016/s0014-2999(01)01297-3. [DOI] [PubMed] [Google Scholar]
- Miralbell R, et al. Nausea and vomiting in fractionated radiotherapy: a prospective on-demand trial of tropisetron rescue for nonresponders to metoclopramide. Eur. J. Cancer. 1995;31A:1461–1464. doi: 10.1016/0959-8049(95)00208-z. [DOI] [PubMed] [Google Scholar]
- Mitchell D. Experiments on neophobia in wild and laboratory rats: a reevaluation. J. Comp. Physiol. Psychol. 1976;90:190–197. doi: 10.1037/h0077196. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK. Poison induced pica in rats. Physiol. Behav. 1976;17:691–697. doi: 10.1016/0031-9384(76)90171-2. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Krusemark ML, Hafner D. Pica: a species relevant behavioural assay of motion sickness in the rat. Physiol. Behav. 1977a;18:125–130. doi: 10.1016/0031-9384(77)90103-2. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Winter W, Morisaki CM. Conditioned taste aversions accompanied by geophagia: evidence for the occurrence of “psychological” factors in the etiology of pica. Psychosom. Med. 1977b;39:401–412. [PubMed] [Google Scholar]
- Mokri B. Spontaneous low cerebrospinal pressure/volume headaches. Curr. Neurol. Neurosci. Rep. 2004;4:117–124. doi: 10.1007/s11910-004-0025-5. [DOI] [PubMed] [Google Scholar]
- Money KE. Motion sickness. Physiol. Rev. 1970;50:1–35. doi: 10.1152/physrev.1970.50.1.1. [DOI] [PubMed] [Google Scholar]
- Morrow GR, Andrews PL, Hickok JT, Stern R. Vagal changes following cancer chemotherapy: implications for the development of nausea. Psychophysiology. 2000;37:378–384. [PubMed] [Google Scholar]
- Morrow GR, Hickok JT, Andrews PL, Stern RM. Reduction in serum cortisol after platinum based chemotherapy for cancer: a role for the HPA axis in treatment-related nausea? Psychophysiology. 2002a;39:491–495. doi: 10.1017.S0048577202991195. [DOI] [PubMed] [Google Scholar]
- Morrow GR, Roscoe JA, Hickok JT, Andrews PR, Matteson S. Nausea and emesis: evidence for a biobehavioral perspective. Support. Care Cancer. 2002b;10:96–105. doi: 10.1007/s005200100294. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Yamakuni H, Nakayama A, Maeda Y, Imazumi K, Matsuo M, Mutoh S. Diphenidol has no actual broad antiemetic activity in dogs and ferrets. J. Pharmacol. Sci. 2004;96:301–306. doi: 10.1254/jphs.fpj04035x. [DOI] [PubMed] [Google Scholar]
- Noble RL. Observations on various types of motion causing vomiting in animals. Can. J. Res. 1945;23:212–225. doi: 10.1139/cjr45e-023. [DOI] [PubMed] [Google Scholar]
- Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J. Comp. Neurol. 1988;273:207–223. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- Nowlis GH, Frank ME, Pfaffmann C. Specificity of acquired aversions to taste qualities in hamsters and rats. J. Comp. Physiol. Psychol. 1980;94:932–942. doi: 10.1037/h0077809. [DOI] [PubMed] [Google Scholar]
- Nussey SS, Hawthorn J, Page SR, Ang VT, Jenkins JS. Responses of plasma oxytocin and arginine vasopressin to nausea induced by apomorphine and ipecacuanha. Clin. Endocrinol. (Oxford) 1988;28:297–304. doi: 10.1111/j.1365-2265.1988.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Page SR, Ang VT, Jackson R, White A, Nussey SS, Jenkins JS. The effect of oxytocin infusion on adenohypophyseal function in man. Clin. Endocrinol. (Oxford) 1990;32:307–313. doi: 10.1111/j.1365-2265.1990.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci. Biobehav. Rev. 1995;19:143–157. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Parker LA, Kwiatkowska M, Burton P, Mechoulam R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew) Psychopharmacology (Berlin) 2004;171:156–161. doi: 10.1007/s00213-003-1571-2. [DOI] [PubMed] [Google Scholar]
- Paton JF, Li YW, Deuchars J, Kasparov S. Properties of solitary tract neurons receiving inputs from the sub-diaphragmatic vagus nerve. Neuroscience. 2000;95:141–153. doi: 10.1016/s0306-4522(99)00416-9. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes—an investigation of the physiological activity of the cerebral cortex. Anrep GV, translator. New York, USA: Dover Publications Inc.; 1927. p. 430. Dover Edition 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagini R, Bianchi PA. Effect of morphine on gastroesophageal reflux and transient lower esophageal sphincter relaxation. Gastroenterology. 1997;113:409–414. doi: 10.1053/gast.1997.v113.pm9247457. [DOI] [PubMed] [Google Scholar]
- Perera AD, Verbalis JG, Mikuma N, Majumdar SS, Plant TM. Cholecystokinin stimulates gonadotropin-releasing hormone release in the monkey (Macaca mulatta) Endocrinology. 1993;132:1723–1728. doi: 10.1210/endo.132.4.8462472. [DOI] [PubMed] [Google Scholar]
- Pezzolla F, Riezzo G, Maselli MA, Giorgio I. Gastric electrical dysrhythmia following cholecystectomy in humans. Digestion. 1991;49:134–139. doi: 10.1159/000200712. [DOI] [PubMed] [Google Scholar]
- Phillips TD. Dietary clay in the chemoprevention of aflatoxininduced disease. Toxicol. Sci. 1999;52:118–126. doi: 10.1093/toxsci/52.suppl_1.118. [DOI] [PubMed] [Google Scholar]
- Phillips TD, Sarr AB, Grant PG. Selective chemisorption and detoxification of aflatoxins by phyllosilicate clay. Nat. Toxins. 1995;3:204–213. doi: 10.1002/nt.2620030407. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA. Relationship between vomiting and taste aversion learning in the ferret: studies with ionizing radiation, lithium chloride, and amphetamine. Behav. Neural Biol. 1992;58:83–93. doi: 10.1016/0163-1047(92)90291-b. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA, Lee J. Intragastric copper sulphate produces a more reliable conditioned taste aversion in vagotomized rats than in intact rats. Behav. Neural Biol. 1985;44:364–373. doi: 10.1016/s0163-1047(85)90664-8. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA, Bakarich AC, Chedester AL, Lee J. Angiotensin II-induced taste aversion learning in cats and rats and the role of the area postrema. Physiol. Behav. 1986a;36:1173–1178. doi: 10.1016/0031-9384(86)90496-8. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA, Chedester AL, Lee J. Role of the area postrema in radiation-induced taste aversion learning and emesis in cats. Physiol. Behav. 1986b;37:815–818. doi: 10.1016/0031-9384(86)90189-7. [DOI] [PubMed] [Google Scholar]
- Reid RM. Cultural and medical perspectives on geophagia. Med. Anthropol. 1992;13:337–351. doi: 10.1080/01459740.1992.9966056. [DOI] [PubMed] [Google Scholar]
- Reynolds RD, Binder HJ, Miller MB, Chang WW, Horan S. Pagophagia and iron deficiency anemia. Ann. Intern. Med. 1968;69:435–440. doi: 10.7326/0003-4819-69-3-435. [DOI] [PubMed] [Google Scholar]
- Riezzo G, Pezzolla F, Darconza G, Giorgio I. Gastric myoelectrical activity in the first trimester of pregnancy: a cutaneous electrogastrographic study. Am. J. Gastroenterol. 1992;87:702–707. [PubMed] [Google Scholar]
- Risinger FO, Brown MM. Genetic differences in nicotine-induced conditioned taste aversion. Life Sci. 1996;58:223–229. doi: 10.1016/0024-3205(96)00051-3. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. DBA/2J mice develop stronger lithium chloride-induced conditioned taste and place aversions than C57BL/6J mice. Pharmacol. Biochem. Behav. 2000;67:17–24. doi: 10.1016/s0091-3057(00)00310-5. [DOI] [PubMed] [Google Scholar]
- Root-Bernstein R, Root-Bernstein M. Honey, mud, and maggots, and other medical marvels. UK: Pan Books, Macmillan Publishers Ltd.; 2000. [Google Scholar]
- Roselle HA. Association of laundry starch and clay ingestion with anemia in New York City. Arch. Intern. Med. 1970;125:57–61. [PubMed] [Google Scholar]
- Rowe JW, Shelton RL, Helderman JH, Vestal RE, Robertson GL. Influence of the emetic reflex on vasopressin release in man. Kidney Int. 1979;16:729–735. doi: 10.1038/ki.1979.189. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Andrews PLR. Mechanisms of acute, delayed, and anticipatory emesis induced by anticancer therapies. In: Hesketh PJ, editor. Management of Nausea and Vomiting in Cancer and Cancer Treatment. Sudbury, MA: Jones and Bartlett; 2005. pp. 15–65. [Google Scholar]
- Rudd JA, Ngan MP, Wai MK. 5-HT3 receptors are not involved in conditioned taste aversions induced by 5-hydroxytryptamine, ipecacuanha or cisplatin. Eur. J. Pharmacol. 1998;352:143–149. doi: 10.1016/s0014-2999(98)00359-8. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Yamamoto K, Yamatodani A, Takeda N. Differential action of ondansetron and dexamethasone to modify cisplatin-induced acute and delayed kaolin consumption (“pica”) in rats. Eur. J. Pharmacol. 2002;454:47–52. doi: 10.1016/s0014-2999(02)02472-x. [DOI] [PubMed] [Google Scholar]
- Saeki M, Sakai M, Saito R, Kubota H, Ariumi H, Takano Y, Yamatodani A, Kamiya H. Effects of HSP-117, a novel tachykinin NK1-receptor antagonist, on cisplatin-induced pica as a new evaluation of delayed emesis in rats. Jpn. J. Pharmacol. 2001;86:359–362. doi: 10.1254/jjp.86.359. [DOI] [PubMed] [Google Scholar]
- Santucci D, Corazzi G, Francia N, Antonelli A, Aloe L, Alleva E. Neurobehavioural effects of hypergravity conditions in the adult mouse. NeuroReport. 2000;11:3353–3356. doi: 10.1097/00001756-200010200-00018. [DOI] [PubMed] [Google Scholar]
- Santucci D, Francia N, Aloe L, Alleva E. Neurobehavioural responses to hypergravity environment in the CD-1 mouse. J. Gravit. Physiol. 2002;9:39–40. [PubMed] [Google Scholar]
- Sarr AB, Mayura K, Kubena LF, Harvey RB, Phillips TD. Effects of phyllosilicate clay on the metabolic profile of aflatoxin B1 in Fischer-344 rats. Toxicol. Lett. 1995;75:145–151. doi: 10.1016/0378-4274(94)03179-b. [DOI] [PubMed] [Google Scholar]
- Schofferman JA. A clinical comparison of syrup of ipecac and apomorphine use in adults. JACEP. 1976;5:22–25. doi: 10.1016/s0361-1124(76)80161-x. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Jacobsen PB, Bovbjerg DH. Role of nausea in the development of aversions to a beverage paired with chemotherapy treatment in cancer patients. Physiol. Behav. 1996;59:659–663. doi: 10.1016/0031-9384(95)02096-9. [DOI] [PubMed] [Google Scholar]
- Selve N, Friderichs E, Reimann W, Reinartz S. Absence of emetic effects of morphine and loperamide in Suncus murinus. Eur. J. Pharmacol. 1994;256:287–293. doi: 10.1016/0014-2999(94)90554-1. [DOI] [PubMed] [Google Scholar]
- Sims DW, Andrews PL, Young JZ. Stomach rinsing in rays. Nature. 2000;404:566. doi: 10.1038/35007149. [DOI] [PubMed] [Google Scholar]
- Singer LK, York DA, Berthoud HR, Bray GA. Conditioned taste aversion produced by inhibitors of fatty acid oxidation in rats. Physiol. Behav. 1999;68:175–179. doi: 10.1016/s0031-9384(99)00172-9. [DOI] [PubMed] [Google Scholar]
- Sipols AJ, Brief DJ, Ginter KL, Saghafi S, Woods SC. Neuropeptide Y paradoxically increases food intake yet causes conditioned flavour aversions. Physiol. Behav. 1992;51:1257–1260. doi: 10.1016/0031-9384(92)90317-u. [DOI] [PubMed] [Google Scholar]
- Smith JC, Jr, Halsted JA. Clay ingestion (geophagia) as a source of zinc for rats. J. Nutr. 1970;100:973–980. doi: 10.1093/jn/100.8.973. [DOI] [PubMed] [Google Scholar]
- Smith WL, Alphin RS, Jackson CB, Sancilio LF. The antiemetic profile of zacopride. J. Pharm. Pharmacol. 1989;41:101–105. doi: 10.1111/j.2042-7158.1989.tb06402.x. [DOI] [PubMed] [Google Scholar]
- Smith JE, Friedman MI, Andrews PL. Conditioned food aversion in Suncus murinus (house musk shrew)— a new model for the study of nausea in a species with an emetic reflex. Physiol. Behav. 2001a;73:593–598. doi: 10.1016/s0031-9384(01)00538-8. [DOI] [PubMed] [Google Scholar]
- Smith JE, Paton JF, Andrews PL. Cardiorespiratory reflexes in a working heart-brainstem preparation of the house musk shrew, Suncus murinus. Auton. Neurosci. 2001b;89:54–59. doi: 10.1016/S1566-0702(01)00251-X. [DOI] [PubMed] [Google Scholar]
- Smotherman WP. Glucocorticoid and other hormonal substrates of conditioned taste aversion. Ann. N.Y. Acad. Sci. 1985;443:126–144. doi: 10.1111/j.1749-6632.1985.tb27068.x. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Maintenance of behaviour by postponement of scheduled injections of nicotine in squirrel monkeys. J. Pharmacol. Exp. Ther. 1983;227:154–159. [PubMed] [Google Scholar]
- Stephan FK, Smith JC, Fisher E. Profound conditioned taste aversion induced by oral consumption of 2-deoxy-D-glucose. Physiol. Behav. 1999;68:221–226. doi: 10.1016/s0031-9384(99)00179-1. [DOI] [PubMed] [Google Scholar]
- Stern RM, Koch KL, Stewart WR, Lindblad IM. Spectral analysis of tachygastria recorded during motion sickness. Gastroenterology. 1987;92:92–97. doi: 10.1016/0016-5085(87)90843-2. [DOI] [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol. Biochem. Behav. 1993;45:817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Horii A, Uno A, Yamatodani A, Matsunaga T. Neuropharmacological mechanisms of emesis. II. Effects of antiemetic drugs on cisplatin-induced pica in rats. Methods Find. Exp. Clin. Pharmacol. 1995;17:647–652. [PubMed] [Google Scholar]
- Tanihata S, Oda S, Nakai S, Uchiyama T. Antiemetic effect of dexamethasone on cisplatin-induced early and delayed emesis in the pigeon. Eur. J. Pharmacol. 2004;484:311–321. doi: 10.1016/j.ejphar.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Tierson FD, Olsen CL, Hook EB. Nausea and vomiting of pregnancy and association with pregnancy outcome. Am. J. Obstet. Gynecol. 1986;155:1017–1022. doi: 10.1016/0002-9378(86)90337-6. [DOI] [PubMed] [Google Scholar]
- Tonini M, De GR, De PF. Progress with novel pharmacological strategies for gastro-oesophageal reflux disease. Drugs. 2004;64:347–361. doi: 10.2165/00003495-200464040-00001. [DOI] [PubMed] [Google Scholar]
- Torii Y, Shikita M, Saito H, Matsuki N. X-irradiationinduced emesis in Suncus murinus. J. Radiat. Res. (Tokyo) 1993;34:164–170. doi: 10.1269/jrr.34.164. [DOI] [PubMed] [Google Scholar]
- Troncon LE, Thompson DG, Ahluwalia NK, Barlow J, Heggie L. Relations between upper abdominal symptoms and gastric distension abnormalities in dysmotility like functional dyspepsia and after vagotomy. Gut. 1995;37:17–22. doi: 10.1136/gut.37.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada H, Hirose T, Yokoyama A, Kurita Y. Randomised comparison of ondansetron plus dexamethasone with dexamethasone alone for the control of delayed cisplatin-induced emesis. Eur. J. Cancer. 2001;37:2398–2404. doi: 10.1016/s0959-8049(01)00326-4. [DOI] [PubMed] [Google Scholar]
- Ueno T, Chen JD. Vomiting and gastric electrical dysrhythmia in dogs. Scand. J. Gastroenterol. 2004;39:344–352. doi: 10.1080/00365520310008601. [DOI] [PubMed] [Google Scholar]
- Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci. 1987;41:513–518. doi: 10.1016/0024-3205(87)90229-3. [DOI] [PubMed] [Google Scholar]
- Ueno S, Matsuki N, Saito H. Suncus murinus as a new experimental model for motion sickness. Life Sci. 1988;43:413–420. doi: 10.1016/0024-3205(88)90520-6. [DOI] [PubMed] [Google Scholar]
- Uneyama H, Niijima A, Tanaka T, Torii K. Receptor subtype specific activation of the rat gastric vagal afferent fibres to serotonin. Life Sci. 2002;72:415–423. doi: 10.1016/s0024-3205(02)02271-3. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Antistress pattern induced by oxytocin. News Physiol. Sci. 1998;13:22–25. doi: 10.1152/physiologyonline.1998.13.1.22. [DOI] [PubMed] [Google Scholar]
- Van Lawick-Goodall H, Van Lawick-Goodall J. Innocent Killers. London, UK: Colloins; 1970. [Google Scholar]
- Vavilova NM, Kassil VG. Conditioned reflex taste aversion during dog ontogeny. Zh. Vyssh. Nerv. Deiat. Im I.P. Pavlova. 1984;34:662–668. [PubMed] [Google Scholar]
- Vig MM. Nicotine poisoning in a dog. Vet. Hum. Toxicol. 1990;32:573–575. [PubMed] [Google Scholar]
- Villablanca JR, Harris CM, Burgess JW, de AI. Reassessing morphine effects in cats: I. Specific behavioural responses in intact and unilaterally brain-lesioned animals. Pharmacol. Biochem. Behav. 1984;21:913–921. doi: 10.1016/s0091-3057(84)80073-8. [DOI] [PubMed] [Google Scholar]
- Watson PJ, Hawkins C, McKinney J, Beatey S, Bartles RR, Rhea K. Inhibited drinking and pica in rats following 2-deoxy-Dglucose. Physiol. Behav. 1987;39:745–752. doi: 10.1016/0031-9384(87)90260-5. [DOI] [PubMed] [Google Scholar]
- Weigel MM, Weigel RM. Nausea and vomiting of early pregnancy and pregnancy outcome. An epidemiological study. Br. J. Obstet. Gynaecol. 1989;96:1304–1311. doi: 10.1111/j.1471-0528.1989.tb03228.x. [DOI] [PubMed] [Google Scholar]
- White J. Sensory innervation of the viscera. Res. Publ. Assoc. 1943;23:373–390. [Google Scholar]
- Wilpizeski CR, Lowry LD, Green SJ, Smith BD, Jr, Melnick H. Subjective concomitants of motion sickness: quantifying rotationinduced illness in squirrel monkeys. Otolaryngol.-Head Neck Surg. 1987;97:433–440. doi: 10.1177/019459988709700501. [DOI] [PubMed] [Google Scholar]
- Woods SC, Figlewicz DP, Madden L, Porte D, Jr, Sipols AJ, Seeley RJ. NPY and food intake: discrepancies in the model. Regul. Pept. 1998;75–76:403–408. doi: 10.1016/s0167-0115(98)00095-0. [DOI] [PubMed] [Google Scholar]
- Wu M, Harding RK, Hugenholtz H, Kucharczyk J. Emetic effects of centrally administered angiotensin II, arginine vasopressin and neurotensin in the dog. Peptides. 1985;6 Suppl. 1:173–175. doi: 10.1016/0196-9781(85)90028-2. [DOI] [PubMed] [Google Scholar]
- Xu LH, Koch KL, Summy-Long J, Stern RM, Seaton JF, Harrison TS, Demers LM, Bingaman S. Hypothalamic and gastric myoelectrical responses during vection-induced nausea in healthy Chinese subjects. Am. J. Physiol. 1993;265:E578–E584. doi: 10.1152/ajpendo.1993.265.4.E578. [DOI] [PubMed] [Google Scholar]
- Xu X, Brining DL, Chen JD. Effects of vasopressin and long pulse-low frequency gastric electrical stimulation on gastric emptying, gastric and intestinal myoelectrical activity and symptoms in dogs. Neurogastroenterol. Motil. 2005;17:236–244. doi: 10.1111/j.1365-2982.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav. Brain Res. 1994;65:123–137. doi: 10.1016/0166-4328(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Matsunaga S, Matsui M, Takeda N, Yamatodani A. Pica in mice as a new model for the study of emesis. Methods Find. Exp. Clin. Pharmacol. 2002a;24:135–138. doi: 10.1358/mf.2002.24.3.802297. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takeda N, Yamatodani A. Establishment of an animal model for radiation-induced vomiting in rats using pica. J. Radiat. Res. (Tokyo) 2002b;43:135–141. doi: 10.1269/jrr.43.135. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ngan MP, Takeda N, Yamatodani A, Rudd JA. Differential activity of drugs to induce emesis and pica behaviour in Suncus murinus (house musk shrew) and rats. Physiol. Behav. 2004;83:151–156. doi: 10.1016/j.physbeh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res. Bull. 1998;47:395–406. doi: 10.1016/s0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Smail JA, Stocker SD, Card JP. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience. 1999;90:1501–1513. doi: 10.1016/s0306-4522(98)00554-5. [DOI] [PubMed] [Google Scholar]
- You CH, Chey WY, Lee KY, Menguy R, Bortoff A. Gastric and small intestinal myoelectric dysrhythmia associated with chronic intractable nausea and vomiting. Ann. Intern. Med. 1981;95:449–451. doi: 10.7326/0003-4819-95-4-449. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Wu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J. Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]