Abstract
Angiomyolipomas (AMLs), a form of benign mesenchymal hamartoma, arise primarily in the kidneys of patients with or without tuberous sclerosis. Extra-renal AMLs are very rare and are most commonly found in the liver. AMLs of the small intestine are exceedingly rare. Here, a case of a 28-year-old man, who presented with ileal intussusception caused by ileal AML is reported. The clinicopathological and immunohistochemical findings of ileal AMLs are discussed and the literature on small intestinal AMLs is reviewed.
Keywords: Angiomyolipoma, Intussusception, Hamartoma, Ileum, Colectomy
INTRODUCTION
Angiomyolipomas (AMLs), benign mesenchymal tumors, are composed of blood vessels, smooth muscle cells, and mature fat cells. These mesenchymal hamartomas arise primarily in the kidney[1] and extrarenal AMLs are very rare. AMLs of the small intestine are exceedingly rare and, to the best of our knowledge, only three cases have been reported in the literature[2,3]. Herein, a case of ileal AML, manifested by small intestinal intussusception, is presented. This occurred in a 28-year-old man, and was confirmed by microscopic examination and immunohistochemical staining after right hemicolectomy.
CASE REPORT
A 28-year-old man presented to the emergency room complaining of progressive abdominal cramping pain, accompanied by nausea and vomiting for 6 h. He had no significant medical history except for having been admitted with pneumothorax 13 years previously. The pain was located over the right lower quadrant of the abdomen. He had no change in bowel habits, bloody stools, or tenesmus. Physical examination showed a palpable mass over the right lower quadrant with direct tenderness. Increased bowel sounds were noted during auscultation. Laboratory findings were normal; red blood cell count 5.02 × 106/mm3, hemoglobin 15.7g/dL and hematocrit 44.1%, platelets 237 × 103/mm3, and white blood cell count 7700/mm3. Serum analysis was as follows: total protein 8.6 g/dL, aspartate aminotransferase 19 U/L, alanine aminotransferase 13 U/L, blood urea nitrogen 13.5 mg/dL, and creatinine 0.7 mg/dL. An enhanced computed tomography (CT) scan of the abdomen showed ileocolic intussusception caused by an abnormal 3.0-cm soft tissue mass with increased thickness of the wall (Figure 1A). There was no evidence of any other soft tissue mass either in the liver or in the kidney. Colonoscopic examination could not be performed because of severe abdominal pain. The patient underwent surgery with the diagnosis of intussusception. During an exploratory laparotomy, the main polypoid mass was located approximately 60 cm from the ileocecal valve. An ileo-ileal intussusception, about 15 cm in length, was found. This mass protruded into the right colon, including the cecum. A right hemicolectomy was uneventfully performed.
Figure 1.
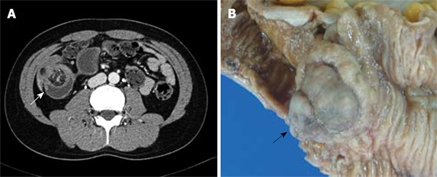
Enhanced abdominal CT and photograph of the pathological specimen. A: A soft tissue mass with fatty-tissue attenuation in the terminal ileum, and with intussusception is shown (arrow); B: Grossly, an approximate 3 cm × 3 cm × 2.5 cm sized polypoid mass was located in the ileum, 60 cm from the ileocecal valve (arrow).
Grossly, an approximately 3 cm × 3 cm × 2.5 cm sized polypoid mass was located in the ileum, 60 cm from the ileocecal valve, and many small polyps were located in the ileum about 25 cm from the ileocecal valve (Figure 1B). The cut surface of the main mass was grayish-yellow in color, with a lobulated appearance and exhibiting no hemorrhage or necrosis. Microscopically, it revealed a mixture of three components: mature adipose tissue, thick-walled vessels, and interspersed areas of spindle-shaped smooth muscle cells (Figure 2A). Immunohistochemically, the smooth muscle cell components of this lesion were consistently positive for α-smooth muscle actin (SMA: Clone 1A4; dilution 1:100; Dako; Figure 2B), desmin (Clone D33; dilution 1:200; Dako; Figure 2C), and vimentin (Clone V9; dilution 1:100; Novocastra). The vascular components were immunoreactive for CD34 (Clone QBEnd/10; dilution 1:50; Novocastra; Figure 2D), but tumor cells were negative for HMB-45 (Novocastra, 1:60) and C-kit (Clone 104D2; dilution, 1:200; Dako). The final pathological diagnosis was AML. The numerous small polyps were diagnosed as lymphoid polyps. The postoperative course was uncomplicated, and the patient was discharged 8 d later. He was seen in the outpatient department for follow-up, and had no tumor recurrence over the subsequent 6 mo.
Figure 2.
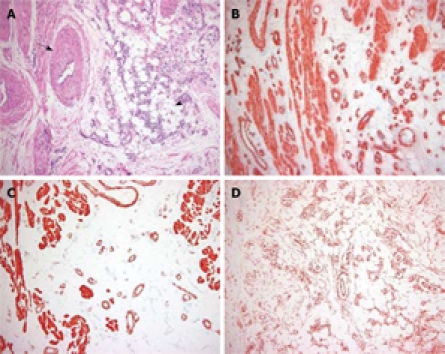
Photomicrograph of the histopathological specimen. A: The specimen was composed of three tissue components: mature adipose tissue (arrowhead) and smooth muscle surrounding thick-walled, medium-sized, vascular channels (arrow) (HE, × 40); B: Immunohistochemistry (IHC) staining showed that the proliferating smooth muscle cells were positive for α-SMA (IHC, × 40); C: Smooth muscle cells showed immunoreactivity for desmin (IHC, × 40); D: The scattered small blood vessels showed immunoreactivity for CD34 (IHC, × 40).
DISCUSSION
AMLs are histologically benign tumors derived from mesenchymal tissue. The vast majority of AMLs arise in the kidney. In 45 to 80% of patients, they are associated with tuberous sclerosis, a multi-systemic disease with autosomal dominant inheritance, and the occasional association with the triad of epilepsy, mental retardation, and adenoma sebaceum[1]. Extrarenal AMLs are very rare and have been reported in the liver[4], nasal cavity[5], vagina[6], spermatic cord[7], skin[8], mediastinum[9], and gastrointestinal (GI) tract, including the colon[10,11–13] and small intestine[2,3]. AMLs arising in the GI tract are extremely uncommon and usually present with melena, anemia, diarrhea, abdominal pain, and may even be clinically asymptomatic[10–13]. Radiological diagnosis of extrarenal AMLs is difficult because of the rarity of the condition. CT is an effective means of imaging and identifying AMLs. Thin-section (3-5 mm) scanning is performed in an attempt to show fatty-tissue attenuation. Sonography can suggest the diagnosis, showing a well-defined hyperechoic lesion, but it is not diagnostic. On magnetic resonance imaging, lesions are bright on T1-weighted images and dark on fat-suppressed images[3]. Surgical excision is the treatment of choice, but inadequate resection may result in rapid local recurrence[14,15]. Microscopically, most AMLs are composed of a variable mixture of mature fat, thick-walled poorly organized blood vessels, and smooth muscle (classic triphasic histology). Rarely, striking degrees of nuclear atypia may be seen in smooth muscle cells, raising the possibility of malignancy. Cells associated with thin-walled, branching vessels with a pattern similar to lymphangioleiomyoma is another variation of the smooth muscle component. The lipomatous component consists typically of mature adipose tissue but may contain vacuolated adipocytes, suggesting lipoblasts, thus mimicking a liposarcoma. The blood vessels are thick-walled and lack the normal elastic content of arteries. AMLs with a prominent vascular component may mimic a vascular malformation. Immunohistochemically, AMLs are characterized by coexpression of melanocytic markers (HMB45) and smooth muscle markers (SMA). However, previously reported cases of intestinal AML[2,3,10–13] showed only focal or no immunoreactivity for HMB45, and the present case was also negative for HMB45. AMLs usually grow slowly. Malignant changes of AML are rare, and only sporadically reported cases have been documented in lesions arising in the kidney. However, to date, AMLs arising in the GI tract have never been reported to have malignant changes.
Only three cases of AML of the small intestine have been previously reported[2,3]. All of the reported cases of small intestinal AMLs are summarized in Table 1. The mean age at diagnosis was 49 years. In renal AMLs, the ratio of male to female patients was 1:9[14]. All of the reported cases of small intestinal AMLs occurred in females, whereas the case presented here occurred in a male. None of the cases were associated with tuberous sclerosis. Three patients with the ileal lesion presented with intussusception, while the patient with the duodenal lesion did not. The tumor sizes ranged from 2 to 4 cm. Also, all four lesions appeared grossly to have a single polypoid pattern.
Table 1.
Clinicopathological data and immunohistochemical staining results in small-intestinal AMLs
| Case No. | 1 | 2 | 3 | 4 |
| Author | Han et al | Toye and Czarnecki | Lin et al | Present case |
| Age (yr) | 60 | 60 | 48 | 28 |
| Gender | Female | Female | Female | Male |
| Tuberous sclerosis | Absence | Absence | Absence | Absence |
| Symptoms | Periumbilical pain | Early satiety | Abdominal pain and bloody stool | Abdominal pain |
| Intussusception | Present | Absent | Present | Present |
| Location | Ileum | Duodenum | Ileum | Ileum |
| Size (cm) | 4 × 4 × 3 | 3.6 × 3.6 | 4 × 4 × 2 | 3 × 3 × 2.5 |
| Gross morphology | Polypoid | Polypoid | Pedunculated polypoid | Polypoid |
| IHC | ||||
| SMA | + | + | + | + |
| DES | + | NA | + | + |
| VIM | + | NA | + | + |
| CD34 | NA | NA | + | + |
| HMB-45 | - | - | +* | - |
| CD117 (c-kit) | NA | NA | +* | - |
DES: Desmin; VIM: Vimentin; +: Positive; -: Negative; NA: Data not available; +*: Positive in few cells.
In conclusion, small intestinal AML is a very rare disease and preoperative diagnosis is difficult. When a patient has abdominal pain with associated ileal intussusception, AML should be considered in the differential diagnosis.
Peer reviewer: Dr. Olivier Detry Department of Abdominal Surgery and Transplantation, University of Liège, CHU Sart Tilman B35, B-4000 Liège, Belgium
S- Editor Li LF L- Editor Cant MR E- Editor Lin YP
References
- 1.Tong YC, Chieng PU, Tsai TC, Lin SN. Renal angiomyolipoma: report of 24 cases. Br J Urol. 1990;66:585–589. doi: 10.1111/j.1464-410x.1990.tb07187.x. [DOI] [PubMed] [Google Scholar]
- 2.Toye LR, Czarnecki LA. CT of duodenal angiomyolipoma [corrected] AJR Am J Roentgenol. 2002;178:92. doi: 10.2214/ajr.178.1.1780092. [DOI] [PubMed] [Google Scholar]
- 3.Lin CY, Chen HY, Jwo SC, Chan SC. Ileal angiomyolipoma as an unusual cause of small-intestinal intussusception. J Gastroenterol. 2005;40:200–203. doi: 10.1007/s00535-004-1527-2. [DOI] [PubMed] [Google Scholar]
- 4.Nonomura A, Mizukami Y, Kadoya M. Angiomyolipoma of the liver: a collective review. J Gastroenterol. 1994;29:95–105. doi: 10.1007/BF01229084. [DOI] [PubMed] [Google Scholar]
- 5.Gatalica Z, Lowry LD, Petersen RO. Angiomyolipoma of the nasal cavity: case report and review of the literature. Head Neck. 1994;16:278–281. doi: 10.1002/hed.2880160312. [DOI] [PubMed] [Google Scholar]
- 6.Chen KT. Angiomyolipoma of the vagina. Gynecol Oncol. 1990;37:302–304. doi: 10.1016/0090-8258(90)90353-m. [DOI] [PubMed] [Google Scholar]
- 7.Castillenti TA, Bertin AP. Angiomyolipoma of the spermatic cord: case report and literature review. J Urol. 1989;142:1308–1309. doi: 10.1016/s0022-5347(17)39068-7. [DOI] [PubMed] [Google Scholar]
- 8.Argenyi ZB, Piette WW, Goeken JA. Cutaneous angiomyolipoma. A light-microscopic, immunohistochemical, and electron-microscopic study. Am J Dermatopathol. 1991;13:497–502. [PubMed] [Google Scholar]
- 9.Bertrand G, Bidabe MC, George P, Dubin P, Touzard C. [Angiomyolipoma of the central mediastinum. An apparently undescribed entity] Ann Chir. 1984;38:679–681. [PubMed] [Google Scholar]
- 10.Maluf H, Dieckgraefe B. Angiomyolipoma of the large intestine: report of a case. Mod Pathol. 1999;12:1132–1136. [PubMed] [Google Scholar]
- 11.Maesawa C, Tamura G, Sawada H, Kamioki S, Nakajima Y, Satodate R. Angiomyolipoma arising in the colon. Am J Gastroenterol. 1996;91:1852–1854. [PubMed] [Google Scholar]
- 12.Chen JS, Kuo LJ, Lin PY, Changchien CR. Angiomyolipoma of the colon: report of a case and review of the literature. Dis Colon Rectum. 2003;46:547–549. doi: 10.1007/s10350-004-6598-x. [DOI] [PubMed] [Google Scholar]
- 13.Hikasa Y, Narabayashi T, Yamamura M, Fukuda Y, Tanida N, Tamura K, Ohno T, Shimoyama T, Nishigami T. Angiomyolipoma of the colon: a new entity in colonic polypoid lesions. Gastroenterol Jpn. 1989;24:407–409. doi: 10.1007/BF02774348. [DOI] [PubMed] [Google Scholar]
- 14.Friis J, Hjortrup A. Extrarenal angiomyolipoma: diagnosis and management. J Urol. 1982;127:528–529. doi: 10.1016/s0022-5347(17)53897-5. [DOI] [PubMed] [Google Scholar]
- 15.Blute ML, Malek RS, Segura JW. Angiomyolipoma: clinical metamorphosis and concepts for management. J Urol. 1988;139:20–24. doi: 10.1016/s0022-5347(17)42276-2. [DOI] [PubMed] [Google Scholar]


