Figure 1. Schematic representation of the self-inactivating lentiviral vector and experimental design.
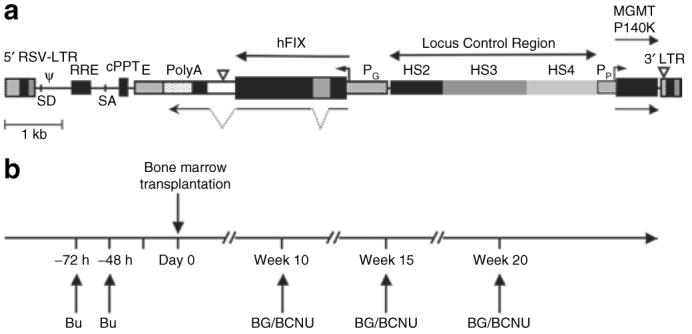
(a) RT9-hFIX-SI-MGMT vector encodes human factor IX (hFIX) cDNA with a truncated hFIX intron placed between the first and second exon sequences of hFIX under the transcriptional control of the β-globin promoter (PG), the 3′ β-globin enhancer (E) and β-globin locus control region (LCR); and the human phosphoglycerate kinase promoter (PP) and methylguanine methyltransferase (MGMT) P140K mutant gene is also included. (b) Experimental design for nonmyeloablative hematopoietic stem cell-based gene transfer and in vivo drug selection. Basically, C57BL/6, FIX-/- recipient mice were conditioned by administration of Busulfex, intraperitoneally 20 mg/kg, twice at 72 and 48 hours before engraftment. Bone marrow cells from syngeneic donor mice were then transduced overnight using RT9-hFIX-SI-MGMT vector. Three MGMT selections were performed using BG/BCNU at indicated time intervals. BCNU, 1,3-bis(2-chloroethyl)-1-nitrosourea; BG, O6-benzylguanine; cPPT, central polypurine tract; LTR, long terminal repeat.
