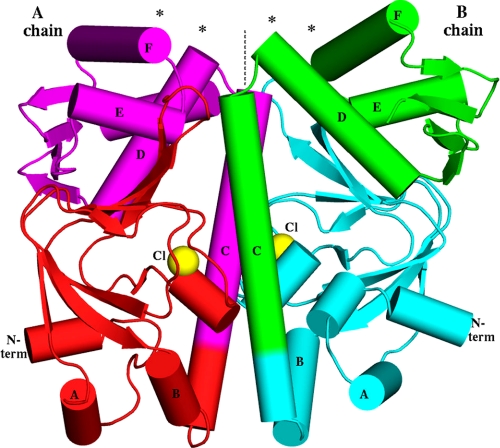
FIGURE 1.
Overall shape of the MtbCRP homodimer. The molecular dyad is vertical (dashed line). Asterisks show the DNA-binding regions at the N termini (N term) of the D and F helices. The locations of the observed chloride ions, coinciding with the phosphate moiety of the cAMP-binding pocket, are shown as yellow spheres. Note that the B chain has an extra short helix in its N-domain, whereas the A chain has an extra helix (the C-terminal helix G, behind D and E in this view) in the C-domain. Because the view direction is perpendicular to the dyad, the image would have approximate mirror symmetry if the dimer were two-fold symmetric, but in fact there are many deviations from mirror symmetry, e.g. the purple E helix is much closer to the dyad than the green E helix is.