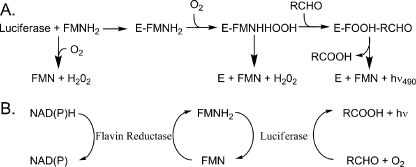
FIGURE 1.
The bioluminescence reaction in vitro and in vivo. A, simplified in vitro bioluminescent reaction mechanism. The isoalloxazine ring of the flavin is oxygenated at position C-4a prior to formation of the aldehyde hemiacetal (47). The resolution of this tetrahedral intermediate leads to the population of an excited state emitter and ultimately light emission (2, 44). B, in vivo, and in the coupled assay in vitro, FMN is reduced by an oxidoreductase enzyme via the oxidation of NADH or NAD(P)H (10). Subsequently, FMNH2 is transferred to bacterial luciferase either by diffusion or by tunneling for the light emitting reaction.