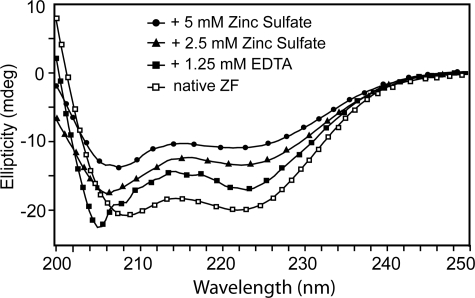
FIGURE 2.
CD spectroscopy and titration with EDTA and ZnSO4 of recombinant Andes virus G1 tail CCHC-region (residues 543–599), which was expressed and purified under native conditions, showed that Zn2+-binding is required for the proper folding of this domain. Native G1 tail zinc finger domain showed a folded CD spectrum (open squares). Titration with an excess of EDTA resulted to an unfolded peak (at 204 nm) and reduced the helical peak (at 222 nm) (closed squares). The addition of 2.5 and 5 mm ZnSO4 yielded folded CD spectra (triangles and circles).