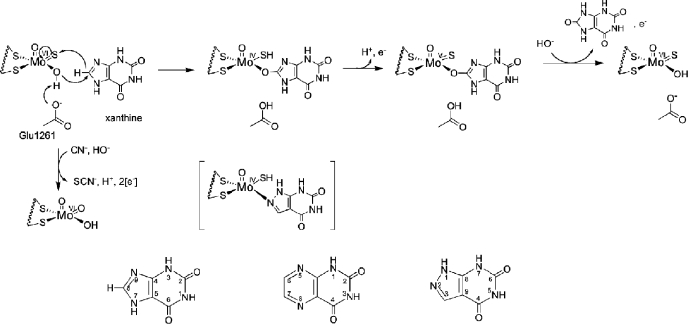
FIGURE 1.
The catalytic mechanism at the molybdenum site of xanthine oxidase. Shown is the hypothesized orientation of xanthine during catalysis. Also shown is the MoV state that gives rise to the well studied “very rapid” EPR signal. The structure in brackets is that of the complex of reduced enzyme with the inhibitor alloxanthine. Inactivation of the enzyme by KCN results from replacement of the planar Mo=S by oxygen from solvent water, forming an unreactive LMoVIO2(OH) form of the active site. Also shown is the numbering scheme for purines, pteridines, and ayrazolopyrimidines, as exemplified by xanthine, lumazine, and alloxanthine, respectively.