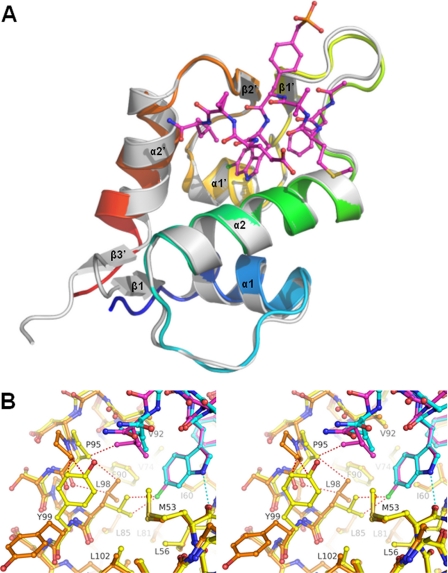
FIGURE 2.
The complexes HdmX-compound 2 and Hdm2-compound 2 have different tilt angles and modified positions for their helices α2′. A chlorine substituent from a ligand at the bottom of the Trp pocket of HdmX can drastically modify the shape of the Leu pocket of HdmX (i.e. there is a cross-talk between these two pockets for HdmX, mediated by Leu98). A, ribbon drawing showing the complex between HdmX (color ramped from the N terminus is in blue to the C terminus is in red) and compound 2 (ball-and-stick-model with carbons in magenta) superposed with Hdm2-compound 2 (white, ligand not shown; PDB entry code 2GV2). B, superposition of the complexes HdmX-compound 1 (color coding as in Fig. 1A, i.e. HdmX with carbons in yellow, compound 1 with carbons in cyan) and HdmX-compound 2 (HdmX with carbons in brown, compound 2 with carbons in magenta, and chlorine in green) zoomed in on the Leu and Trp pockets. The hydrogen bond between the indole nitrogen and CO-Met53 is indicated as a cyan dotted line. Steric clashes that would occur without movement of selected atoms are shown as red dotted lines. To avoid a steric clash with the 6-chlorine substituent of compound 2, Leu98 has to adopt a different side chain orientation (the distance between 6-chlorine and CD1-Leu98 would be 2.9 Å without reorientation). This leads to a movement of Pro95 and a side chain flip of Tyr99, which drastically changes the shape of the Leu pocket. As a consequence, e.g. the Leu side chain of compound 2 can penetrate more deeply (CD2 moves by 1.5 Å into the Leu pocket). The diagram is programmed for stereo viewing.