FIGURE 1.
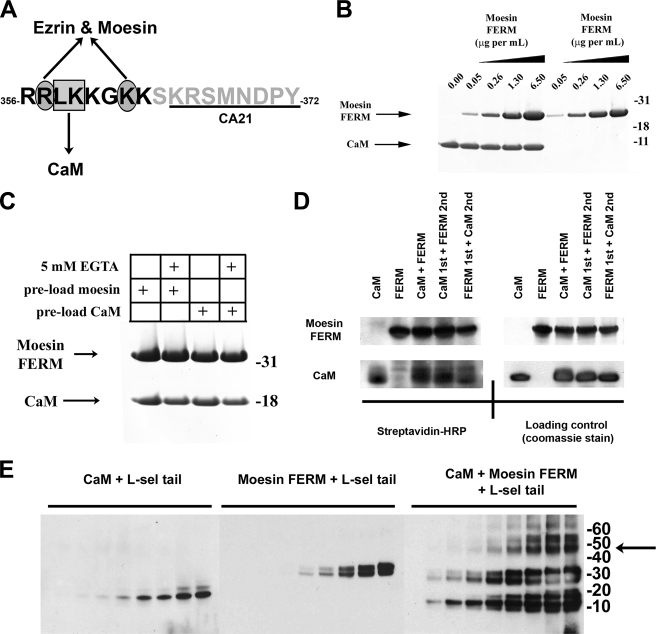
Moesin FERM and CaM bind non-competitively with the cytoplasmic tail of l-selectin. A, single letter amino acid sequence of the cytoplasmic tail of L-selectin. Black letters define the polybasic, membrane proximal domain. The box and ovals depict amino acid residues that have been previously shown to contribute to binding CaM and ERM, respectively (see Refs. 5, 6, 9 for more detail). Underlined region of the L-selectin tail marks the epitope recognized by the CA21 monoclonal antibody. B, Coomassie-stained polyacrylamide gel showing the relative binding of moesin FERM or CaM to the L-selectin beads, which were pre-saturated with (lanes 1-5) or without (lanes 6-9) CaM. 5 μg/ml of CaM was used to preload the beads prior to incubation with increasing amounts of moesin FERM domain. Coomassie-stained gels are representative of three independent experiments. C, binding of moesin FERM domain and CaM to the cytoplasmic tail of L-selectin is calcium-independent. The L-selectin beads were preloaded with either moesin FERM (lanes 1 and 2) or CaM (lanes 3 and 4). Preloaded beads were then incubated with CaM and moesin FERM, respectively. Binding reaction was supplemented either with (lanes 2 and 4) or without (lanes 1 and 3) 5 mm EGTA. Bound proteins were resolved on polyacrylamide gels and subsequently stained with Coomassie Blue. Gel is representative of three independent experiments. D, biotin transfer of SBED from the L-selectin tail to either moesin FERM domain or CaM is equal and independent of pre-mixing (see supplemental Fig. S1 and “Materials and Methods” for more information of SBED biotin transfer procedure). In brief, 3.6 μm SBED-conjugated L-selectin tail was mixed with 4.6 μm CaM and/or moesin FERM at room temperature for 30 min. In mixing experiments, a 30-min gap was left between adding proteins, which was deemed ample time for the first protein to bind to the L-selectin tail. The left-hand top and bottom panels represent PVDF transfer membranes developed with 1 μg/ml streptavidin-horseradish peroxidase. The right-hand top and bottom panels represent the same PVDF membranes from the left-hand panels, which were subsequently stained with Coomassie Blue to show relative abundance of CaM and moesin FERM used in the experiment (loading control), and is representative of three independent experiments. E, equal concentrations of CaM (4.6 μm) or moesin FERM (4.6 μm) were mixed individually or together with increasing amounts of soluble L-selectin tail peptide (i.e. 0, 1.72, 3.44, 6.88, 13.75, 27.50, 55, 110, and 220 μm). Protein products were cross-linked with DSS, resolved on polyacrylamide gels, and transferred to a PVDF membrane for Western blotting with CA21 monoclonal anti-L-selectin tail antibody. Shifts in molecular masses of the L-selectin tail corresponded to the molecular mass of CaM (18 kDa), moesin FERM (30 kDa), or a mixture of the two (50 kDa). The arrow to the right of the molecular weight markers denotes the higher molecular weight complexes that likely correspond to a 1:1:1 stoichiometry between the tail of L-selectin, CaM, and moesin FERM. The Western blot is representative of three independent experiments.