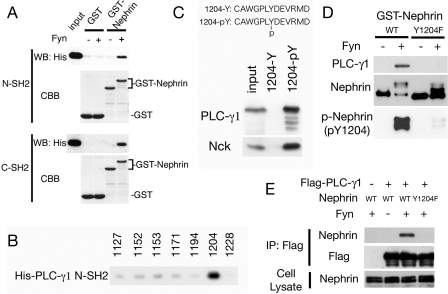
FIGURE 4.
PLC-γ1 directly binds to phosphorylated Nephrin Tyr(P)-1204. A, recombinant GST or GST-Nephrin-CD was bound to glutathione-Sepharose beads and incubated with or without Fyn. After washing, the beads were incubated with recombinant His-tagged N- or C-SH2 domain of PLC-γ1, and bound proteins were immunoblotted with anti-His antibody. CBB, Coomassie Brilliant Blue staining. WB, Western blot. B, phosphorylated Nephrin peptides were immobilized to SulfoLink coupling gel to pull down His-PLC-γ1-N-SH2. PLC-γ1-N-SH2 specifically bound to phospho-Tyr-1204. C, phosphorylated or nonphosphorylated Nephrin Tyr-1204 peptides were immobilized to coupling gel and incubated with HEK293T cell lysates. The bound proteins were immunoblotted with anti-PLC-γ1 and anti-Nck. D, GST-Nephrin-CD wild type or Y1204F were phosphorylated by Fyn and immobilized to glutathione-Sepharose. The beads were incubated with HEK293T lysates, and bound proteins were analyzed by Western blotting for PLC-γ1.E, HEK293T cells were transfected with indicated vectors, and anti-FLAG immunoprecipitates (IP) and cell lysates were analyzed by Western blotting for FLAG and Nephrin.