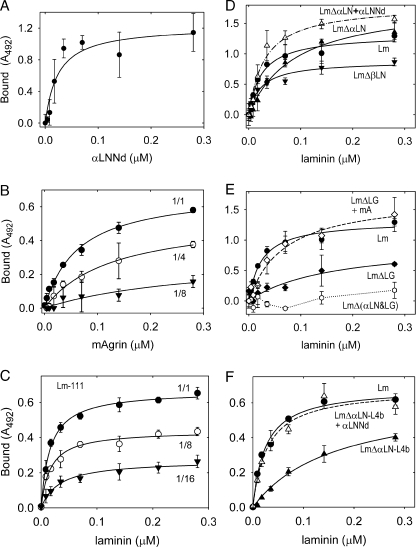
FIGURE 5.
Protein binding to galactosyl sulfatide. The indicated components (single or equimolar pairs) were incubated at the indicated concentrations in lipid-coated wells. Binding was detected with antibodies to laminin (FLAG epitope-horseradish peroxidase), αLNNd (Myc), or mAgrin (agrin). The average and S.D. values (n = 3) and fitted regressions for simple binding (solid and dashed lines) are shown in each graph. Panel A, binding of αLNNd to galactosyl-3-sulfate-ceramide. Panel B, binding of mAgrin to galactosyl-3-sulfate-ceramide undiluted (1/1) or diluted (1/4, 1/8) in galactosyl-ceramide. Panel C, binding of (wt) laminin to galactosyl-3-sulfate-ceramide undiluted (1/1) or diluted (1/8, 1/16) in galactosyl-ceramide. Panel D, Binding of Lm (closed circles), LmΔαLN (closed triangles), LmΔαLN + αLNNd (open triangles), and LmΔβLN (closed inverted triangles). Panel E, Binding of Lm (closed circles), LmΔLG (closed diamonds), LmΔLG + mA (open diamonds), and LmΔ(αLN&LG) (open circles). Panel F, Binding of Lm (closed circles), LmΔαLN-L4b (closed triangles), and LmΔαLN-L4b +αLNNd (open triangles). Contributions fromαLNNd, mAgrin, and laminin LG and αLN domains were detected. Dilution of the sulfated galactosyl ceramide in galactosyl ceramide resulted in decreased binding for mAgrin and laminin. Coupling of αLNNd to LmΔαLN, αLNNd to LmΔαLN-L4b, and mA to LmΔLG increased the laminin affinities.