Abstract
Amidated peptides are critically involved in many physiological functions. Genetic deletion of peptidylglycine α-amidating monooxygenase (PAM), the only enzyme that can synthesize these peptides, is embryonically lethal. The goal of the present study was the identification of physiological functions impaired by haploinsufficiency of PAM. Regulation of the hypothalamic-pituitary-thyroid axis and body temperature, functions requiring contributions from multiple amidated peptides, were selected for evaluation. Based on serum T4 and pituitary TSH-β mRNA levels, mice heterozygous for PAM (PAM+/−) were euthyroid at baseline. Feedback within the hypothalamic-pituitary-thyroid axis was impaired in PAM+/− mice made hypothyroid using a low iodine/propylthiouracil diet. Despite their normal endocrine response to cold, PAM+/− mice were unable to maintain body temperature as well as wild-type littermates when kept in a 4 C environment. When provided with additional dietary copper, PAM+/− mice maintained body temperature as well as wild-type mice. Pharmacological activation of vasoconstriction or shivering also allowed PAM+/− mice to maintain body temperature. Cold-induced vasoconstriction was deficient in PAM+/− mice. This deficit was eliminated in PAM+/− mice receiving a diet with supplemental copper. These results suggest that dietary deficiency of copper, coupled with genetic deficits in PAM, could result in physiological deficits in humans.
Thermoregulation and vasoconstriction are deficient in mice with limited peptide amidating monooxygenase, a cuproenzyme; dietary copper reverses both deficits.
In most systems, it is the interaction of genetic traits and environmental factors that determines whether physiological deficits occur. In mouse and Drosophila, peptidylglycine α-amidating monooxygenase (PAM), a copper-dependent enzyme, is the only means of producing the plethora of amidated peptides involved in homeostasis (1,2). Genetic elimination of PAM results in embryonic lethality. PAM null mouse embryos do not survive past embryonic d 15, exhibiting severe edema and thinning of the aorta and carotid arteries (1).
Our initial study showed that PAM heterozygous mice (PAM+/−), although viable, differed from wild-type (WT) littermate controls in adiposity and their ability to handle a glucose load (1). Regulation of the hypothalamic-pituitary-thyroid (HPT) axis and body temperature involves multiple amidated peptides acting in multiple systems (3,4,5,6,7,8,9,10,11,12). We used PAM+/− mice to test the hypothesis that haploinsufficiency of PAM results in physiological deficits in these processes. Because the function of PAM is absolutely dependent on copper (13,14,15,16), we assessed the ability of dietary copper supplementation to reverse the deficits caused by decreased levels of PAM.
PAM is the only enzyme capable of amidating peptides, a posttranslational modification that is crucial for bioactivity (1,2,17). Amidated peptides generally bind more tightly to their receptors and have longer half-lives than peptides with a free C terminus (18,19,20). The hydroxylation of peptidylglycine substrates by PAM is dependent on the binding of copper to two sites in the monooxygenase domain (14,15,21,22,23,24,25). The redox properties of copper are essential for its interaction with molecular oxygen, and copper cannot be replaced by any other metal. In addition to its catalytic role, PAM plays important roles in vesicular trafficking (16), secretion and cytoskeletal structure (26).
Amidated peptides are numerous and include TRH, neuropeptide Y, and vasopressin (17,19). Amidated peptides make critical contributions to a diversity of physiological processes including HPT axis regulation (27,28), thermoregulation (7), and cardiovascular function (29,30). Hypothyroidism increases pituitary levels of several amidated peptides [e.g. neuropeptide Y (4), substance P (5), vasoactive intestinal peptide (6)] and increases levels of PAM mRNA and enzymatic activity (3,31,32). We therefore selected the pituitary response to hypothyroidism as one test for physiological deficits in PAM+/− mice.
TRH (7), neuropeptide Y (8,9,10,11), and vasopressin (12) also play critical roles in regulating body temperature. A point mutation in carboxypeptidase E, the enzyme that precedes PAM in the peptide biosynthetic pathway, diminished the ability of mice to regulate body temperature in the cold (7). Based on these observations, we also assessed thermoregulation in PAM+/− mice.
We present data demonstrating that production of an amidated peptide is compromised in the hypothalamus of PAM+/− mice; these mice are deficient in their pituitary response to hypothyroidism. We also show that PAM+/− mice are deficient in their ability to maintain body temperature in the cold. The deficit in temperature regulation involves failure to constrict peripheral vessels. When supplemented with copper, PAM+/− mice regained the ability to maintain body temperature in the cold.
Materials and Methods
Animal care and use
Male and female PAM+/− mice (crossed with C57BL/6J mice for more than 10 generations) and C57BL/6J WT mice (2–5 months old) were used in these experiments; male PAM+/− mice were bred with female C57BL/6J mice from The Jackson Laboratory (Bar Harbor, ME), and weanlings genotyped. All protocols conformed to University of Connecticut Health Center Animal Care Committee guidelines.
Genotyping
PAM+/− and WT DNA samples were analyzed using platinum Taq polymerase (Invitrogen, Carlsbad, CA) (supplemental Fig. 1, published as supplemental data on The Endocrine Society's Journals Online web site at http://endo.endojournals.org): mouse PAM-rI (5′-ACC ACA GCT ATA CAC GCA AAT GCC TTG C-3′), Neo f1 (5′-CTG CGT GCA ATC CAT CTT GTT CAA TG-3′); mouse PAM exon3-f2 (GAA TGC CTT GGT ACC ACC AGA CCC ATC ACT CC). PCR conditions were: 94 C for 2 min; denaturation, 94 C for 30 sec; anneal, 68 C for 1 min; elongate, 72 C for 35 sec; repeat from step 2, 30 cycles; final elongation for 5 min. PCR products analyzed on 2% agarose gels yield a single band at 200 nt (WT) or bands at 400 and 200 nt (PAM+/−) (supplemental Fig. 1).
RNA isolation
Pituitary and brown adipose RNA was extracted using Trizol (Invitrogen); RNA (5 μg) was converted into cDNA using Superscript reverse transcriptase (Invitrogen).
PAM RT-PCR
cDNA (1–2.5% of sample) was amplified using primers in exon 3 (Exon3-f2), exon 5 [exon5-rev (5′-CCC AGT GGA TGA GGG CAT GTT GC-3′)], exon 6 [exon-6rev (5′-GGA GCC GGG TGG GGG GAG CAT TTC-3′)], and the neomycin sequence [Neo-f2 (5′-CTG CGT GCA ATC CAT CTT GTT CAA TGCCG-3′)] (supplemental Fig. 2). PCR conditions were as above, 30 cycles for exon 3/exon 6 and 34 cycles for Neo/Exon 6.
PAM assays and Western blots
Pituitaries, paraventricular nuclei (PVN) and preoptic areas (POA) from PAM+/− and WT littermates were dissected using standard landmarks (33) and individually homogenized (13); 0.1–1 μg protein was assayed for PAM activity (13). Optimal activity required including 10 μm CuSO4 (hypothalamus) or 7.5 μm CuSO4 (pituitary). Western analyses were performed using primary antibody to the exon A region of PAM-1 (Ab 629; 1:1000) (1); this antibody recognizes full-length PAM-1, monofunctional peptidylglycine α-hydroxylating monooxygenase (PHM), and monofunctional peptidyl-α-hydroxyglycine α-amidating lyase (PAL). Images were acquired and quantified using GeneTools (Syngene, Frederick, MD); only nonsaturated images were used for quantification.
TRH RIAs
The POAs and PVNs from individual mice were homogenized in 2 n acetic acid/2 mm EGTA/protease inhibitors, heated to 95 C for 5 min, and frozen. Lyophilized supernatants were assayed for amidated TRH and glycine instead of amide (TRH-Gly) (7).
Pharmacologically induced hypothyroidism
Adult male mice were divided into four groups by genotype and diet and kept on a normal diet or a low-iodine diet containing 6-n-propyl-2-thiouracil (PTU) (Harlan Teklad, Indianapolis, IN; diet 97061) for 14 d (34). Body weights were monitored, with no difference between genotypes (not shown). Tail vein blood was collected after 4 and 7 d of treatment. After 14 d, animals were killed by decapitation; trunk blood was collected. Hypothalami were extracted for RIA of TRH and TRH-Gly. Pituitaries were harvested for quantitative PCR analysis of TSHβ and PAM transcripts.
T4 assays
Serum T4 (ng/ml) was determined using the total T4 Coat-A-Count kit (Siemens Healthcare Diagnostics, New York, NY; TKT41); results were calculated using the logit-log method (35). After 4 d of PTU treatment, serum levels of T4 were reduced to half in both PAM+/− and WT mice; after 14 d of PTU treatment, serum levels of T4 fell below the detection limit of the assay (0.01 ng/ml; supplemental Table 1), as expected (3,31,34).
Quantitative PCR for TSH and PAM
cDNA prepared from individual pituitaries was analyzed for PAM, TSHβ, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts using specific gene expression assay kits (Applied Biosystems, Foster City, CA). Expression levels were normalized to those measured in pooled WT pituitaries.
Thermoregulation with dietary copper supplementation
For 14 d, male mice received drinking water supplemented with 300 ppm CuSO4 · 5H2O [70 ppm Cu2+, a dose that avoids toxicity in rodents (36)] or deionized water, with normal mouse chow. No differences in body weight were observed (not shown). Core body temperature was monitored with a rectal probe (Harvard Apparatus, Holliston, MA). All mice were handled for 5 d before exposure to cold; each mouse was placed into a precooled (4 C) cage with no bedding or drinking water. Core body temperature was monitored 1 h before placement at 4 C and after 40, 80, and 120 min of exposure to 4 C.
Norepinephrine RIA
Trunk blood was collected into 25 μl 250 μm EDTA. Plasma was separated after a 15-min centrifugation at 14,000 rpm and assayed using the noradrenaline-adrenaline research RIA (Rocky Mountain Diagnostics, Colorado Springs, CO; BA 5400).
Corticosterone RIAs
Plasma corticosterone was determined using an RIA kit (MP Biomedicals, Costa Mesa, CA ImmunoChem 07-120102). Recommended reagent volumes were cut in half so that plasma samples could be assayed in duplicate.
Uncoupling protein (UCP)-1 expression in brown adipose tissue
Brown adipose tissue was homogenized in Trizol (Invitrogen) for preparation of RNA. Primers used were: actin forward, 5′-GCT GGT CGT CGA CAACGGCTC C-3′; actin reverse, 5′-CAA ACA TGA TCT GGG TCA TCT TTT CAC GG-3′; GAPDH forward, 5′-TTG TCA GCA ATG CAT CCT GCA CCA C-3′; GAPDH reverse, 5′-GGG AGT TGC TGT TGA AGT CAC AGG A-3′; UCP1 forward, 5′-GAC GTC CCC TGC CAT TTA CGT-3′; and UCP1 reverse, 5′-CAA TCC ACT GTC TGT CTG GAC-3′. The three pairs of primers were amplified separately using an annealing temperature of 50 C for UCP1 (19 cycles) and GAPDH (19 cycles) and 56 C for actin (23 cycles). Wet weights of brown adipose tissue ranged from 39 mg to 73 mg in WT mice and from 47 mg to 120 mg in PAM+/− mice, as expected (37,38).
Pharmacological manipulation of cold sensitivity
A 5-hydroxytryptamine-1A antagonist, WAY-100635 (1 mg/kg; Sigma Aldrich, St. Louis, MO), was used to induce shivering (39,40) upon cold exposure. An α1-adrenergic agonist, phenylephrine (2 mg/kg ip; Sigma Aldrich), was used to induce vasoconstriction (41). For both drugs, rectal temperature was monitored every 40 min over the course of a 120-min cold exposure. Ketamine (10 mg/100 g ip; Wyeth Pharmaceuticals) was used to inhibit shivering (42); anesthetized mice were placed in direct contact with ice packs stored at −20 C and rectal temperature recorded every 30 sec during the 5 min of ice pack contact.
Laser Doppler velocimetry
Vasoconstriction was monitored using a laser Doppler probe (Moor Instruments, Devon, UK; DRT4) to record changes in red blood cell (RBC) flux in the tail vein of anesthetized (1.5% isoflurane in air-oxygen mixture) littermates. Mice received a 2 mg/kg ip injection of phenylephrine or saline. Changes in RBC flux are expressed as a percent increase over baseline (43,44).
Statistical analysis
One-way ANOVAs were used to compare PAM+/− and WT mice at baseline or to selectively compare the effect of pharmacological treatments within either WT or PAM+/− mice. Two-way ANOVAs were used to determine the overall effect of PTU treatment on levels of serum T4 as well as TSHβ and PAM in the pituitary. Two-way ANOVAs were also used to determine the overall effect of cold exposure on serum levels of T4, norepinephrine, corticosterone, and brown adipose UCP1. ANOVAs of mixed design with both the within-subject factor of either temperature or laser Doppler readings over time and the between-subject factors of genotype and pharmacological treatment were used to evaluate changes in body temperature and RBC flux. A single star indicates significance levels between 0.05 and 0.001, two stars, significance levels between 0.001 and 0.0005; three stars, significance levels less than 0.0005.
Results
Verification of PAM+/− model of haploinsufficiency
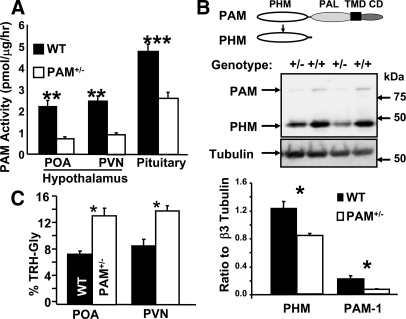
Because genetic elimination of PAM was incompatible with life and PAM+/− mice displayed alterations in adiposity and glucose metabolism (1), we undertook a more detailed examination of the PAM+/− mice. We focused on the hypothalamus, rich in amidated peptides. Baseline levels of PAM activity and protein were assessed in the POA and PVN, critical to the HPT axis and thermoregulation (3,4,5,6,7,8,9,10,11,12), and the pituitary gland (Fig. 1A). PAM activity in the POA, PVN, and pituitary of PAM+/− mice was no more than half of WT levels. Based on immunoblot analyses, levels of intact PAM-1 and soluble PHM in the PVN of PAM+/− mice were reduced compared with WT levels (Fig. 1B).
Figure 1.
Verification of animal model of PAM haploinsufficiency. Extracts from individual mice were used to quantify PAM enzymatic activity (A) in the POA, PVN, and pituitary gland of PAM+/− (n = 4–6) and WT (n = 4–6) mice as described in Materials and Methods; error bars, sem. One-way ANOVAs demonstrated the following levels of significance: POA, P = 0.002; PVN, P = 0.001; pituitary, P < 0.0005. PAM levels in individual PVN extracts (30 μg protein) from PAM+/− and WT mice (n/group = 4) were determined by immunoblot analysis using a rabbit polyclonal antibody to exon A, which detects full-length PAM-1 and cleaved PHM and peptidyl-α-hydroxyglycine α-amidating lyase (PAL) (B). Data for PAM were normalized to levels of β3-tubulin, which were determined after stripping the blot; error bars, sem. One-way ANOVAs revealed a significant difference between genotypes: PHM, P = 0.008; PAM-1 P = 0.023. Levels of TRH-Gly and amidated TRH were assessed in POA and PVN extracts from PAM+/− (n = 7 or 8) and WT (n = 7 or 8) mice (C). Raw data (nanograms peptide per milligram protein) are presented in supplementary Table 2. Percent TRH-Gly was calculated using the sum of TRH-Gly plus TRH as the total; error bars, sem. One-way ANOVAs revealed the following levels of statistical significance: POA, P = 0.001; PVN, P = 0.002. Asterisk, See Statistical analysis for P-values.
If levels of PAM limit production of amidated peptides, a 2-fold reduction in activity should result in accumulation of glycide extended PAM substrates. TRH-Gly and amidated TRH RIAs were used to quantify these peptides in POA and PVN extracts (Fig. 1C and supplemental Table 2). Substantial amounts of TRH-Gly were detected in both the POA and PVN of WT mice. In PAM+/− mice, the percentage of TRH-Gly almost doubled in both hypothalamic nuclei. In PAM+/− mice, there was no compensatory increase in expression of PAM from the WT allele and the ability of hypothalamic neurons to convert TRH-Gly into TRH was diminished. It is not yet clear whether similar deficits occur in the processing of other peptidyl-Gly intermediates.
Regulation of the HPT axis is deficient in PAM+/− mice
Because many amidated peptides are up-regulated within the pituitary in response to hypothyroidism (4,5,6), we hypothesized that regulation of the HPT axis would be deficient in PAM+/− mice. To test this hypothesis, pharmacological hypothyroidism was produced with a low-iodine diet containing 6-n-propyl-2-thiouracil for 14 d (34). The low iodine/PTU diet effectively induced hypothyroidism in both PAM+/− and WT mice as verified by quantification of serum T4 (supplemental Table 1) (3,31,34). With this dietary treatment, the growth rates of both WT and PAM+/− mice did not differ from controls (data not shown).
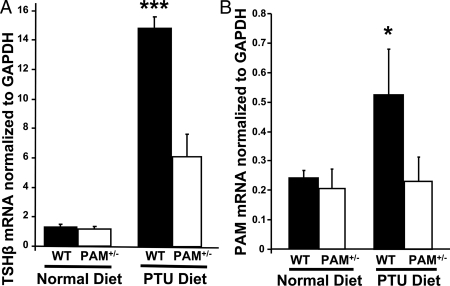
To assess the response of the HPT axis to pharmacologically induced hypothyroidism, RNA was prepared from the pituitaries of WT and PAM+/− mice. Levels of TSHβ mRNA were assessed by quantitative PCR (Fig. 2A). In mice kept on the normal diet, levels of TSHβ mRNA were equivalent in PAM+/− and WT mice. As expected (3), pituitary TSHβ mRNA levels in WT mice increased 14-fold in response to hypothyroidism. In contrast, only a 6-fold increase in TSHβ mRNA levels was observed in PAM+/− mice kept on the low iodine/PTU diet. This blunted increase in TSHβ expression in PAM+/− mice demonstrated that feedback regulation of the HPT axis is deficient.
Figure 2.
HPT axis regulation is altered in PAM+/− mice. Pituitary levels of TSH-β (A) and PAM (B) mRNA (normalized to GAPDH) were determined in euthyroid and hypothyroid animals by quantitative PCR. PAM+/− (n/group = 8–9) and WT (n/group = 8 each) mice were kept on the low-iodine/PTU diet or a normal diet for 14 d; error bars, sem. Two independent sets of animals were examined; data shown are a combination of both experiments (total = 42; n/group = 7–9). A two-way ANOVA revealed a significant effect of low-iodine/PTU diet on the levels of TSH (P < 0.0005) and a significant interaction (i.e. a difference in the effect of the diet) between genotype and the low-iodine/PTU diet (P < 0.0005; A). WT mice showed a more substantial increase in TSH mRNA to hypothyroidism than PAM+/− mice. The pituitary levels of PAM mRNA increased in response to hypothyroidism in WT mice but not PAM+/− mice. A second two-way ANOVA revealed a significant effect of low-iodine/PTU diet on the levels of PAM (P = 0.023) and a significant interaction between genotype and the low-iodine/PTU diet (P = 0.05; B). Asterisk, See Statistical analysis for P-values.
TRH-Gly and TRH were measured in PVN and POA extracts prepared from WT and PAM+/− mice on both diets (supplemental Fig. 1). Hypothyroidism did not change the percentage of TRH-Gly or the total amount of TRH in either hypothalamic nucleus in PAM+/− or WT mice. Under both conditions, TRH-Gly accounted for a greater percentage of the total TRH plus TRH-Gly in PAM+/− than WT mice.
Regulation of pituitary PAM expression is deficient in PAM+/− mice
PAM expression in the rat anterior pituitary was previously shown to increase in response to hypothyroidism (3,31). A similar response was observed in WT mice; pituitary PAM mRNA levels increased 2-fold in response to low iodine/PTU diet-induced hypothyroidism (Fig. 2B). This increase in pituitary PAM expression was absent in PAM+/− mice. Although PAM activity was reduced in the pituitaries of PAM+/− mice (Fig. 1A), levels of PAM mRNA determined by quantitative PCR were indistinguishable in PAM+/− and WT pituitaries (Fig. 2B). The commercial primers used for quantitative PCR analysis of PAM mRNA detect both the normal PAM transcript and a transcript containing the neomycin resistance gene (supplemental Fig. 2); this transcript, which cannot encode PAM, appears to be quite stable.
Thermoregulation is impaired in PAM+/− mice and rescued by copper supplementation
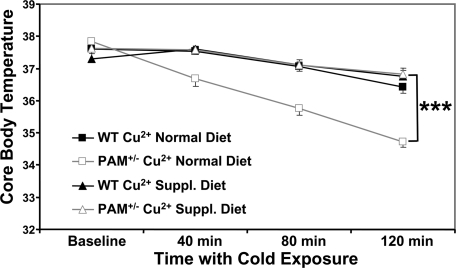
Many amidated peptides are essential for the maintenance of body temperature (7,8,10,11,12) and other deficits in peptide synthesis impair thermoregulation (7). Baseline body temperature did not differ in WT and PAM+/− mice (37.6 ± 0.2 vs. 37.8 ± 0.1 C; Fig. 3). PAM+/− and WT mice were subjected to a 120-min cold exposure, and their core body temperatures were measured every 40 min (Fig. 3). For WT mice, core body temperature declined only 1 C over 120 min. In contrast, PAM+/− mouse core body temperature dropped 3 C.
Figure 3.
Inability of PAM+/− mice to regulate body temperature is reversed by dietary copper. Core body temperatures of PAM+/− (n = 8, 9) and WT (n = 9, 11) mice with and without dietary copper supplementation (Cu2+ Suppl.; n/group = 8–11) were monitored every 40 min in response to a 120-min exposure to 4 C; error bars, sem. An ANOVA of this mixed design revealed significant main effects of both genotype and copper supplementation (P < 0.0005) and a significant interaction between genotype and copper supplementation (P < 0.0005). Only the PAM+/− mice showed a significant change in their body temperature in response to supplementation of dietary copper. Asterisk, See Statistical analysis for P-values.
Knowing that PAM is a limiting factor in the production of at least one neuropeptide (Fig. 1C), we hypothesized that its ability to function might be enhanced by increasing copper availability. Both copper binding sites in its active site must be metallated for catalysis to occur (14,15,25). Dietary supplementation of copper protects the vasculature and increases brain catecholamines (45,46). WT and PAM+/− mice were provided with deionized water or water supplemented with 70 ppm copper for 2 wk before cold exposure (Fig. 3). Without altering body weight, the copper supplement fully rescued the thermoregulatory deficit identified in PAM+/− mice; core body temperature declined only 1 C after 120 min at 4 C. Temperature regulation in WT mice was not altered by dietary copper supplementation, linking the effects of copper supplementation to PAM. We therefore explored the cause of the thermoregulatory deficit in PAM+/− mice.
Endocrine responses to cold exposure are appropriately elevated in PAM+/− mice
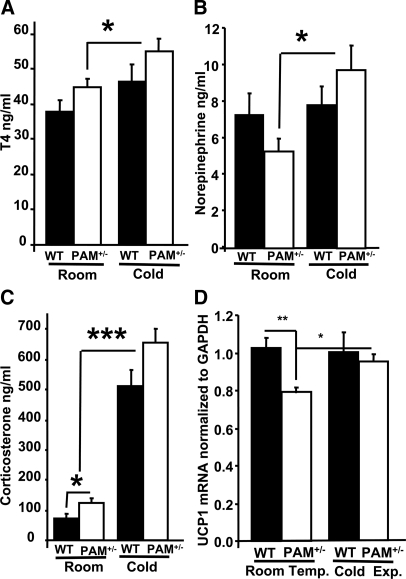
We assessed the endocrine response to cold exposure to better understand the thermoregulatory deficit in PAM+/− mice. Plasma was harvested from mice kept at room temperature or at 4 C for 120 min. Three endocrine components of thermoregulation, T4 (7,47,48), norepinephrine (48,49,50), and corticosterone (51), were assessed. Baseline levels of T4 and norepinephrine were not statistically different in PAM+/− and WT mice (Fig. 4, A and B). Baseline levels of corticosterone were increased in PAM+/− mice (Fig. 4C).
Figure 4.
Endocrine responses to cold exposure. Plasma harvested from PAM+/− and WT mice (n/group = 8–11) housed at room temperature or at 4 C for 2 h was assayed for T4 (A), norepinephrine (B), and corticosterone (C); error bars, sem. Univariate analysis of variance revealed the following significance of cold exposure: T4, P = 0.005; norepinephrine, P = 0.032; corticosterone, P < 0.0005. One-way ANOVAs revealed the following PAM+/− responses to cold: T4, P = 0.031; norepinephrine, P = 0.013. An additional one-way ANOVA revealed a significant increase in baseline corticosterone in PAM+/− mice, P = 0.039. D, UCP1 mRNA levels (normalized to GAPDH) were quantified in brown adipose tissue harvested from PAM+/− (n = 6) and WT (n = 6) mice kept at room temperature or 4 C for 120 min. The average level in WT mice housed at room temperature was normalized to 1.0; error bars, sem. In PAM+/− mice, one-way ANOVAs revealed a significant decrease in baseline UCP1 expression (P = 0.004) and a significant increase in cold-stimulated UCP1 expression (P = 0.01). Asterisk, See Statistical analysis for P-values.
In response to cold exposure, levels of T4, norepinephrine, and corticosterone increased significantly in PAM+/− mice (Fig. 4). Whereas serum T4 and norepinephrine in WT mice were not increased by cold exposure, levels of corticosterone were increased significantly. Corticosterone levels in cold exposed PAM+/− mice were significantly higher than in cold exposed WT mice. Given the greater loss in body temperature that occurs in PAM+/− mice, these results show that their endocrine response to cold is appropriately elevated compared with WT littermate controls.
UCP1 expression in brown adipose tissue is reduced at baseline and responds appropriately to cold exposure in PAM+/− mice
To investigate the metabolic component of thermoregulation (52,53), RNA was isolated from brown adipose tissue at room temperature and at the conclusion of the 120 min cold exposure. Baseline levels of UCP1 mRNA were decreased in PAM+/− mice compared with WT littermate controls (Fig. 4D); despite this difference, baseline body temperature was not affected. Cold exposure brought about a significant increase in UCP1 mRNA levels in PAM+/− mice but not WT mice. Similar results were seen when levels of UCP1 were normalized to β-actin (not shown). Taken together, these results show an appropriate thermogenic response to cold exposure in brown adipose tissue of PAM+/− mice, which become significantly colder than WT mice during the 2 h exposure to 4 C.
Pharmacological stimulation of shivering prevents loss of body temperature in PAM+/− mice
The decline in core body temperature observed in PAM+/− mice could reflect a failure to generate heat through shivering, which is difficult to measure in mice. Serotonergic agonists induce hypothermia by decreasing shivering (54), whereas serotonergic antagonists increase shivering (39,40). PAM+/−and WT mice were administered a single ip injection of saline or the serotonergic antagonist WAY-100635 at the onset of cold exposure (Fig. 5A) to pharmacologically induce shivering. The core body temperature of PAM+/− mice given WAY-100635 dropped less than 1 C. In contrast, temperature regulation was unaltered in WT mice after treatment with WAY-100635. Pharmacological activation of shivering enabled PAM+/− mice to maintain body temperature at 4 C without altering the response of WT mice.
Figure 5.
Pharmacological manipulation of shivering and vasoconstriction. A, PAM+/− and WT mice received a single ip injection of the serotonin antagonist WAY-100635 (1 mg/kg) at the onset of a 2-h exposure to 4 C (n/group = 4–8). Average core body temperatures are shown; error bars, sem. An ANOVA of this mixed design revealed a significant interaction between genotype and pharmacological treatment (P < 0.0005), demonstrating that this pharmacological treatment had a significant effect on the body temperature of PAM+/− mice but not WT mice. B, PAM+/− (n = 4–8) and WT (n = 4–8) mice received a single ip injection of ketamine (10 mg/100 g) 3 min before a 5-min exposure to ice packs. PAM+/− and WT mice experienced equivalent levels of hypothermia. C, PAM+/− and WT mice received a single ip injection of the noradrenergic agonist phenylephrine (2 mg/kg) at the onset of a 2-h cold exposure. Average core body temperatures are reported (n/group = 4–8). An ANOVA of this mixed design revealed a significant main effect of genotype (P = 0.029) and a significant interaction between genotype and pharmacological treatment (P < 0.0005). Asterisk, See Statistical analysis for P-values.
General anesthetics inhibit shivering and vasoconstriction in response to cold (42). It was hypothesized that equivalent levels of hypothermia would be induced in anesthetized PAM+/− and WT mice. To test this hypothesis, PAM+/− and WT mice were given a single ip injection of ketamine to inhibit shivering/vasoconstriction at the onset of cold exposure (Fig. 5B). Equivalent levels of hypothermia were induced. Taken together, our data suggest that failure to shiver and/or vasoconstrict in response to cold exposure could contribute to the inability of PAM+/− mice to maintain body temperature.
Pharmacological stimulation of vasoconstriction prevents loss of body temperature in PAM+/− mice
The decline in core body temperature observed in PAM+/− mice might reflect failure to conserve heat through vasoconstriction. A pharmacological approach was taken to investigate this possibility. To induce vasoconstriction, PAM+/− and WT mice were given a single ip injection of the α-adrenergic agonist phenylephrine or saline upon cold exposure (Fig. 5C). Phenylephrine prevented the loss of body temperature observed in saline injected PAM+/− mice and was without effect on WT mice. The ability of phenylephrine to cause vasoconstriction in PAM+/− and WT mice was verified using laser Doppler velocimetry and did not differ (supplemental Fig. 3). As observed for shivering, our data suggest that failure to vasoconstrict in response to the cold could contribute to the inability of PAM+/− mice to maintain body temperature.
Cold-induced vasoconstriction is impaired in PAM+/− mice
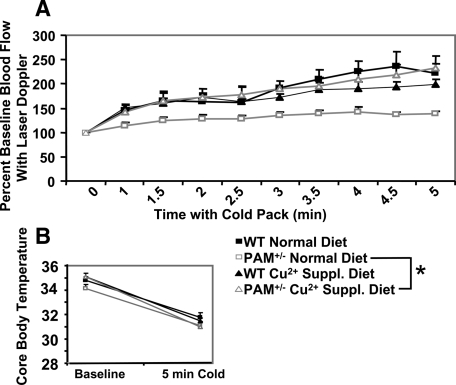
We used laser Doppler velocimetry to determine whether cold exerted a different effect on vasoconstriction in WT and PAM+/− mice. Mice were anesthetized with isoflurane, and a stable tail vein laser Doppler signal was obtained and set to 100%. Ice packs were placed next to the mouse and rectal temperature and laser Doppler signal were recorded over the next 5 min (Fig. 6A). Whereas WT mice more than doubled tail vein blood RBC flux after 5 min of cold exposure, PAM+/− mice showed a blunted increase in tail vein RBC flux. Anesthetized WT and PAM+/− mice showed a similar drop in body temperature after 5 min of ice pack contact (Fig. 6B). These results demonstrate that an impairment in PAM+/− cold-induced vasoconstriction contributes to their inability to maintain body temperature in the cold.
Figure 6.
A, PAM+/− (total n = 15; n/group = 7–8, in white) and WT (total n = 14; n/group = 6– 7, in black) mice were anesthetized with isoflurane and a laser Doppler probe was used to monitor blood flow through their tail veins before and during a 5-min cold exposure (direct contact with ice packs stored at −20 C). The averaged data are presented as a percentage of baseline blood flow, and the error bars indicate the sem. A one-way ANOVA of repeated measures revealed a significant difference separating the genotypes (P < 0.0005). Additional analysis with a two-way ANOVA of repeated measures indicated that 14 d of supplementation (Suppl.) of dietary copper fully rescued the PAM+/− deficit in cold-induced vasoconstriction without altering the WT level of cold-induced vasoconstriction. Specifically, a significant overall three-way interaction between blood flow, genotype, and dietary copper treatment (P = 0.012) was seen. Moreover, statistical analysis of the between subject factors revealed significant interaction between genotype and dietary copper treatment (P = 0.031). B, Core body temperature at baseline and after 5 min of cold exposure are shown.
We next asked whether dietary copper supplementation could reverse the vasoconstriction deficit observed in PAM+/− mice. PAM+/− mice supplemented with 70 ppm copper in their drinking water showed a doubling in tail vein RBC flux after 5 min of cold exposure (Fig. 6A). Copper supplementation did not alter cold-induced vasoconstriction in WT mice. Copper supplementation did not alter the decline in body temperature observed in the anesthetized animals (Fig. 6B). The inability of PAM+/− mice to respond to cold exposure by vasoconstriction is reversed by providing supplemental copper.
Discussion
Levels of PAM limit peptide amidation
For some genes, having only one functional allele has profound physiological and behavioral effects. Haploinsufficiency of single transcription factors (e.g. Nkx2-1 or TBX5) is associated with neurological, thyroid, and pulmonary dysfunction (55). Haploinsufficiency of the vesicular glutamate transporter, vesicular monoamine transporter, Na/K-ATPase α-isoform, or neuregulin 1 in mice results in biochemical deficits, increased anxiety-like and depressive-like behaviors, and deficits in spatial recognition and motor function (56,57,58,59). Mouse strains vary in their response to genetic and experimental manipulations, and it is interesting that PAM is one of the central nervous system genes whose expression varies most widely among strains (60). Single-nucleotide polymorphisms identified in the human PAM gene (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=5066) may affect gene expression or enzymatic activity.
Baseline physiology is altered in PAM+/− mice
At baseline, PAM+/− mice show few behavioral or endocrine deficits; their reproductive and motor abilities are normal (1). They are euthyroid, with normal core body temperature; circulating levels of T4 and pituitary levels of TSHβ mRNA are normal. Plasma corticosterone is elevated and brown adipose levels of UCP1 mRNA are diminished, but fasting plasma glucose is normal (1). The decline in UCP1 expression in PAM+/− mouse brown adipose tissue could be related to a decrease in plasma norepinephrine, which did not reach statistical significance. Further analysis of both the hypothalamic-pituitary-adrenal axis and sympathetic system may reveal additional changes in PAM+/− mice.
Hypothalamic feedback and thermoregulation are altered in PAM+/− mice
Despite their baseline euthyroid status, endocrine feedback in response to hypothyroidism was impaired in PAM+/− mice. Pharmacologically induced hypothyroidism failed to produce the same increase in pituitary TSHβ or PAM mRNA in PAM+/− mice as in WT mice. Whereas both the modest decrease in TRH amidation and in total levels of TRH observed in the PVN and POA of PAM+/− mice may contribute, TRH is unlikely to be the only factor. The pathways through which hypothyroidism produces an increase in pituitary PAM mRNA are unknown. Hypothyroidism induces an increase in transcription of the TSHβ gene (61), but the increase in pituitary PAM mRNA involves mRNA stabilization (31), presumably via increased binding of la autoantigen (32).
When placed at 4 C, the body temperature of PAM+/− mice dropped faster than in WT littermate controls. The endocrine response of the PAM+/− mice to cold exposure was appropriate; levels of T4, norepinephrine, and corticosterone all increased. The higher levels of corticosterone in cold exposed PAM+/− mice may reflect the baseline alterations in hypothalamic-pituitary-adrenal axis regulation that elevate baseline corticosterone.
When placed into a cold environment, PAM+/− mice failed to constrict blood flow in peripheral vessels as effectively as WT animals. Phenylephrine, an α-adrenergic agonist, produced similar amounts of vasoconstriction in PAM+/− and WT mice, indicating that the target vasculature was capable of responding appropriately. Body temperature was restored to WT levels in PAM+/− mice given phenylephrine, demonstrating that a failure to prevent surface heat loss is at least part of the thermoregulatory deficit in PAM+/− mice. Amidated peptides expected to participate in this process include neuropeptide Y, vasopressin, and adrenomedullin. Injection of MK771, a potent peripherally and centrally active TRH agonist (62), did not restore PAM+/− body temperature to normal (not shown), demonstrating that supplementation of this single amidated peptide is not sufficient to rescue the PAM+/− thermogenic deficit.
Lacking a reliable means of assessing shivering in mice, we demonstrated that WAY-100635, a pharmacological agent expected to induce shivering without altering vasoconstriction (39,40,54,63), raised body temperature in PAM+/− mice placed in the cold. Thus, skeletal muscle responds appropriately in PAM+/− mice. A 2-h exposure to cold is not long enough to involve a significant contribution from UCP1 in brown adipose tissue (53).
PAM function is limited by copper availability
Given the complexity of the peptidergic system and the lack of response to MK771, we thought it unlikely that replacement of a single peptide would rescue the deficits identified in PAM+/− mice, so we manipulated dietary copper. Copper availability is known to affect peptide amidation. Symptoms associated with Menkes disease, a genetic deficiency in the transport of copper into the secretory pathway, include severe mental retardation, epilepsy, and hypothermia (64). Amidation of neuropeptides is impaired in Menkes disease (65) and seizures seen in patients with Menkes disease are ameliorated with copper supplementation (66). The pool of copper in humans is small, with a turnover rate of about 2%/d when dietary intake is 2 mg/d; about two thirds of the copper is in the liver and one third elsewhere, including the brain (67,68). A 3-fold drop in dietary copper intake can cause deficiency and a 5-fold increase can cause toxicity. Our data suggest that further study may reveal additional effects caused by mild copper deficiency.
PAM+/− mice receiving supplementary copper regained the ability to maintain body temperature in the cold and regained the ability to control peripheral blood flow when placed into a 4 C environment. Because we do not have an accurate means of assessing PAM activity in situ, we cannot conclude that increased dietary copper resulted in increased PAM enzymatic activity and increased peptide amidation. We do know that the additional dietary copper did not affect thermoregulation in WT mice. Dietary regimens elevating copper within the physiological range (36) are protective against experimentally imposed cardiac hypertrophy (45) and increase catecholamine levels in plasma and brains of ob/ob mice (46). The nutritional literature does not provide a clear answer on optimal levels of dietary copper and has not allowed identification of patients with mild copper deficiency (69,70). Ataxia, myelopathy, and dietary copper deficiency are seen in human patients after gastric bypass surgery (71).
PAM activity cannot be measured accurately in tissue lysates without adding exogenous copper (13). As observed in the atrium (1), copper-optimized in vitro PAM activity in the hypothalamus and the pituitary was decreased to half in PAM+/− mice. Whereas amidation of proopiomelanocortin products in the pituitary was not compromised in PAM+/− mice (1), TRH-Gly accumulated in the hypothalamus. The active site of PAM accommodates all 20 amino acids, but Michaelis constant values for different substrates vary widely (19). Deficiencies in the amidation of peptides like TRH, which terminate with Pro-NH2, would be expected to appear before deficiencies in low Michaelis constant substrates like αMSH (−Val-NH2) or cholecystokinin (−Phe-NH2). An in situ assay that reflected the activity of PAM in the secretory pathway of individual cells would greatly increase our understanding of this system.
Peptidergic signaling: the intersection of genes and environment
PAM requires both copper and ascorbate to support the monooxygenase reaction (14,15). Rodents synthesize ascorbate but are dependent on their diet for copper. Humans express inactive l-gulono-γ-lactone oxidase and cannot synthesize ascorbic acid; both ascorbate and copper must come from the diet (72). Whereas the underlying mechanisms are not yet understood, this work makes it clear that optimal peptidergic function requires adequate levels of both PAM and supplies of dietary copper.
Reduced levels of individual amidated peptides may contribute to specific deficits. In addition, the effects of the integral membrane PAM protein on regulated secretion (26) and cytoskeletal organization (26,73) may also play critical roles. Through its interactions with Kalirin and Trio, Rho guanidine nucleotide exchange factor expressed throughout the central nervous system, changes in PAM expression may alter the balance of basal and stimulated secretion (26). Increased dietary copper may restore the ability to maintain body temperature by ensuring adequate metallation of the monooxygenase domain of PAM. However, copper availability is known to alter the endocytic trafficking of PAM, affecting the ability of this integral membrane protein to return to secretory granules after exocytosis (16). If levels of PAM affect cellular handling of copper, the effects of copper on glutamatergic transmission (74,75) and specific ion channels (76) may also come into play.
Supplementary Material
Acknowledgments
We thank Darlene D'Amato and Yanping Wang for much help in the laboratory, especially with mouse husbandry and genotyping. We also thank Drs. Louise McCullough, Jun Li, and Christine Turtzo for guidance using their laser Doppler. We also thank Dr. Robert E. Schwartz (Merck Research Laboratories, Rahway, NJ) for MK771.
Footnotes
This work was supported by National Institutes of Health Grants DK32949 (to B.A.E. and R.E.M.) and MH080589 (to D.B.-M.).
Disclosure Summary: No authors have any conflicts of interest to declare.
First Published Online November 20, 2008
Abbreviations: GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; HPT, hypothalamo-pituitary-thyroid; NPY, neuropeptide Y; PAM, peptidylglycine α-amidating monooxygenase; PHM, peptidylglycine α-hydroxylating monooxygenase; POA, preoptic area; PTU, 6-n-propyl-2-thiouracil; PVN, paraventricular nucleus; RBC, red blood cell; TRH-Gly, TRH with glycine instead of amide; UCP, uncoupling protein; VP, vasopressin; WT, wild type.
References
- Czyzyk TA, Ning Y, Hsu MS, Peng B, Mains RE, Eipper BA, Pintar JE 2005 Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev Biol 287:301–313 [DOI] [PubMed] [Google Scholar]
- Jiang N, Kolhekar AS, Jacobs PS, Mains RE, Eipper BA, Taghert PH 2000 PHM is required for normal developmental transitions and for biosynthesis of secretory peptides in Drosophila. Dev Biol 226:118–136 [DOI] [PubMed] [Google Scholar]
- Ouafik LH, May V, Saffen DW, Eipper BA 1990 Thyroid hormone regulation of peptidylglycine α-amidating monooxygenase expression in anterior pituitary gland. Mol Endocrinol 4:1497–1505 [DOI] [PubMed] [Google Scholar]
- Jones P, Ghatei M, Steel J, O'Halloran D, Gon G, Legon S, Burrin J, Leonhardt U, Polak J, Bloom S 1989 Evidence for neuropeptide Y synthesis in the rat anterior pituitary and the influence of thyroid hormone status: comparison with vasoactive intestinal peptide, substance p, and neurotensin. Endocrinology 125:334–341 [DOI] [PubMed] [Google Scholar]
- Aronin N, Coslovsky R, Chase K 1988 Hypothyroidism increases substance P concentrations in the heterotropic anterior pituitary. Endocrinology 122:2911–2914 [DOI] [PubMed] [Google Scholar]
- Lam K, Lechan RM, Minamitani N, Segerson TP, Reichlin S 1989 Vasoactive intestinal peptide in the anterior pituitary is increased in hypothyroidism. Endocrinology 124:1077–1083 [DOI] [PubMed] [Google Scholar]
- Nillni EA, Xie W, Mulcahy L, Sanchez VC, Wetsel WC 2002 Deficiencies in pro-thyrotropin-releasing hormone processing and abnormalities in thermoregulation in CPEfat/fat mice. J Biol Chem 277:48587–48595 [DOI] [PubMed] [Google Scholar]
- Woods A, Stock M 1996 Inhibition of brown fat activity during hypothalamic stimulation in the rat. Am J Physiol 270:R605–R613 [DOI] [PubMed] [Google Scholar]
- Hwa J, Witten M, Williams P, Ghibaudi L, Gao J, Salisbury B, Mullins D, Hamud F, Strader C, Parker E 1999 Activation of the NPY Y5 receptor regulates both feeding and energy expenditure. Am J Physiol Regul Integ Comp Physiol 277:R1428–R1434 [DOI] [PubMed] [Google Scholar]
- Pelz K, Dark J 2007 ICV NPY Y1 agonist but not Y5 agonist induces torpor-like hypothermia in cold-acclimated Siberian hamsters. Am J Physiol Regul Integ Comp Physiol 292:R2299–R2311 [DOI] [PubMed] [Google Scholar]
- Dark J, Pelz K 2008 NPY Y1 receptor antagonist prevents NPY-induced torporlike hypothermia in cold-acclimated Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 294:R236–R245 [DOI] [PubMed] [Google Scholar]
- Ye J, Clark M, Colquhoun E 1995 Constant-pressure perfusion of rat hindlimb shows α- and β-adrenergic stimulation of oxygen consumption. Am J Physiol 269:E960–E968 [DOI] [PubMed] [Google Scholar]
- Kolhekar AS, Mains RE, Eipper BA 1997 Peptidylglycine alpha-amidating monooxygenase (PAM): an ascorbate requiring enzyme. Methods Enzymol 279:35–43 [DOI] [PubMed] [Google Scholar]
- Prigge ST, Mains RE, Eipper BA, Amzel LM 2000 New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. Cell Mol Life Sci 57:1236–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert X, Eipper BA, Mains RE, Prigge ST, Blackburn NJ, Amzel LM 2005 The catalytic copper of peptidylglycine α-hydroxylating monooxygenase also plays a critical structural role. Biophys J 89:3312–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De M, Ciccotosto GD, Mains RE, Eipper BA 2007 Trafficking of a secretory granule membrane protein is sensitive to copper. J Biol Chem 282:23362–23371 [DOI] [PubMed] [Google Scholar]
- Eipper BA, Stoffers DA, Mains RE 1992 The biosynthesis of neuropeptides: peptide alpha-amidation. Annu Rev Neurosci 15:57–85 [DOI] [PubMed] [Google Scholar]
- Cuttitta F 1993 Peptide amidation: signature of bioactivity. Anat Rec 236:87–93 [DOI] [PubMed] [Google Scholar]
- Merkler DJ 1994 C-terminal amidated peptides: Production by the in vitro enzymatic amidation of glycine-extended peptides and the importance of the amide to biological activity. Enzyme Microbiol Technol 16:450–456 [DOI] [PubMed] [Google Scholar]
- In Y, Fujii M, Sasada Y, Ishida T 2001 Structural studies on C-amidated amino acids and peptides: structures of hydrochloride salts of C-amidated Ile-Val, Thr, Ser, Met, Trp, Gln, and Arg, and comparison with their C-unamidated counterparts. Acta Crystallog B Struct Sci 203:631–639 [DOI] [PubMed] [Google Scholar]
- Eipper BA, Glembotski CC, Mains RE 1983 Bovine intermediate pituitary α-amidation enzyme: preliminary characterization. Peptides 4:921–928 [DOI] [PubMed] [Google Scholar]
- Jaron S, Mains RE, Eipper BA, Blackburn NJ 2002 The catalytic role of the copper ligand H172 of peptidylglycine α-hydroxylating monooxygenase (PHM): a spectroscopic study of the H172A mutant. Biochemistry 41:13274–13282 [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Bailey WR 1995 Alterations of rat brain peptidyglycine α-amidating monooxygenase and other cuproenzyme activities following perinatal copper deficiency. Proc Soc Exp Biol Med 210:107–116 [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Broderius M 2006 Plasma peptidylglycine α-amidating monooxygenase (PAM) and ceruloplasmin are affected by age and copper status in rats and mice. Comp Biochem Physiol B Biochem Mol Biol 143:360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge ST, Kolhekar AS, Eipper BA, Mains RE, Amzel LM 1997 Amidation of bioactive peptides: the structure of peptidylglycine α-hydroxylating monooxygenase. Science 278:1300–1305 [DOI] [PubMed] [Google Scholar]
- Mains RE, Alam MR, Johnson RC, Darlington DN, Back N, Hand TA, Eipper BA 1999 Kalirin, a multifunctional PAM COOH-terminal domain interactor protein, affects cytoskeletal organization and ACTH secretion from AtT-20 cells. J Biol Chem 274:2929–2937 [DOI] [PubMed] [Google Scholar]
- Yamada M, Saga Y, Shibusawa N, Hirato J, Murakami M, Iwasaki T, Hashimoto K, Satoh T, Wakabayashi K, Taketo M, Mori M 1997 Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc Natl Acad Sci USA 94:10862–10867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikrodhanond AA, Ortiga-Carvalho TM, Shibusawa N, Hashimoto K, Liao XH, Refetoff S, Yamada M, Mori M, Wondisford FE 2006 Dominant role of thyrotropin-releasing hormone in the hypothalamic-pituitary-thyroid axis. J Biol Chem 281:5000–5008 [DOI] [PubMed] [Google Scholar]
- Eto T 2001 A review of the biological properties and clinical implications of adrenomedullin and proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and vasodilating peptides. Peptides 22:1693–1711 [DOI] [PubMed] [Google Scholar]
- Eto T, Samson WK 2001 Adrenomedullin and proadrenomedullin n-terminal 20 peptide: vasodilatory peptides with multiple cardiovascular and endocrine actions. Trends Endocrinol Metab 12:91–93 [DOI] [PubMed] [Google Scholar]
- Fraboulet S, Boudouresque F, Delfino C, Fina F, Oliver C, Ouafik L 1996 Effect of thyroid hormones on peptidylglycine α-amidating monooxygenase gene expression in anterior pituitary gland: transcriptional studies and messenger ribonucleic acid stability. Endocrinology 137:5493–5501 [DOI] [PubMed] [Google Scholar]
- Brenet F, Dussault N, Borch J, Ferracci G, Delfino C, Roepstorff P, Miquelis R, Ouafik L 2005 Mammalian peptidylglycine α-amidating monooxygenase mRNA expression can be modulated by the la autoantigen. Mol Biol Cell 25:7505–7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curras M, Dao J 1998 Developmental plasticity of NR1 and NR2B subunit expression in the supraoptic nucleus of the rat hypothalamus. Brain Res Dev Brain Res 109:1–12 [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Murata Y, Sadow P, Hayashi Y, Seo H, Xu J, O'Malley B, Weiss R, Refetoff S 2002 Steriod receptor coactivator-1 deficiency causes variable alterations in the modulation of T3-regulated transcription of genes in vivo. Endocrinology 143:1346–1352 [DOI] [PubMed] [Google Scholar]
- Davis S, Munson P, Jaffe M, Rodbard D 1980 Radioimmunoassay data processing with a small programmable calculator. J Immunoassay 1:15–25 [DOI] [PubMed] [Google Scholar]
- Herbert C, Elwell M, Travlos G, Fitz C, Bucher J 1993 Subchronic toxicity of cupric sulfate administered in drinking water and feed of mice and rats. Fund Appl Toxicol 21:461–475 [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS 2000 Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M, Taberner P 2001 Effects of acute and chronic ethanol administration on the response of mouse adipose tissue hormone-sensitive lipase to α2-adrenoreceptor activation by UK 14304. Alcohol Alcohol 36:381–387 [DOI] [PubMed] [Google Scholar]
- Hoffman J, Brown J, Sirlin E, Benoit A, Gill W, Harris M, Darnall R 2007 Activation of 5-HT1A receptors in the paragigantocellularis lateralis decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol 293:R518–R527 [DOI] [PubMed] [Google Scholar]
- Brown J, Sirlin E, Benoit J, Darnall R 2008 Activation of 5-HT1A receptors in medullary raphe disrupts sleep and decreases shivering. Am J Physiol Regul Integr Comp Physiol 294:R884–R894 [DOI] [PubMed] [Google Scholar]
- Zarrindast M, Sadeghi S, Sahebgharani M 2003 Influence of α-adrenoceptor agonists and antagonists on imipramine-induced hypothermia in mice. Pharmacol Toxicol 93:48–53 [DOI] [PubMed] [Google Scholar]
- Sagir O, Gulhas N, Toprak H, Yucel A, Begec Z, Erosy O 2007 Control of shivering during regional anaesthesia: prophylactic ketamine and granisetron. Acta Anaesthesiol Scand 51:44–49 [DOI] [PubMed] [Google Scholar]
- Cho S, Park E, Zhou P, Frys K, Ross M, Iadecola C 2005 Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab 25:493–501 [DOI] [PubMed] [Google Scholar]
- Pijls N, van Son J, Kirkeeide R, De Bruyne B, Gould K 1993 Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 86:1354–1367 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, Tyagi S, Eaton J, Saari J, Kang Y 2007 Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med 204:657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Chen M, Wang C, Lin P 1995 Dietary copper supplementation increases the catecholamine levels in genetically obese (ob/ob) mice. Biol Trace Elem Res 50:243–247 [DOI] [PubMed] [Google Scholar]
- Perello M, Stuart R, Vaslet C, Nillni E 2007 Cold exposure increases the biosynthesis and proteolytic processing of prothyrotropin-releasing hormone in the hypothalamic paraventricular nucleus via β-adrenoreceptors. Endocrinology 148:4952–4964 [DOI] [PubMed] [Google Scholar]
- Silva E 2006 Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86:435–464 [DOI] [PubMed] [Google Scholar]
- Cavadas C, Waeber B, Pedrazzini T, Grand D, Aubert J, Buclin T, Grouzmann E 2007 NPY Y1 receptor is not involved in the hemodynamic response to an acute cold pressor test in mice. Peptides 28:315–319 [DOI] [PubMed] [Google Scholar]
- Gray S, Yamaguchi N, Vencova P, Sherwood N 2002 Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology 143:3946–3954 [DOI] [PubMed] [Google Scholar]
- Zalutskaya A, Arai M, Bounoutas G, Abou-Samra A 2007 Impaired adaptation to repeated restraint and decreased response to cold in urocortin 1 knockout mice. Am J Physiol Endocrinol Metab 293:E256–E263 [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J 2004 Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359 [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD 1997 Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature 387:94–97 [DOI] [PubMed] [Google Scholar]
- Martin K, Phillips I, Hearson M, Prow M, Heal D 1992 Characterization of 8-OH-DPAT-induced hypothermia in mice as a 5-HT1A autoreceptor response and its evaluation as a model to selectively identify antidepressants. Br J Pharmacol 107:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J, Seidman C 2002 Transcription factor haploinsufficiency; when half is not enough. J Clin Invest 109:451–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordera R, Totterdell S, Wojcik S, Brose N, Elizalde N, Lasheras B, Del Rio J 2007 Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGlut 1). Eur J Neurosci 25:281–290 [DOI] [PubMed] [Google Scholar]
- Fukui M, Rodriguiz R, Zhou J, Jiang S, Phillips L, Caron M, Wetsel W 2007 Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci 27:10520–10529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley A, Williams M, Schaefer T, Bohanan C, Neumann J, Behbehani M, Vorhees C, Lingrel J 2007 Deficiency in Na, K-ATPase α-isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci 27:616–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Duffy L, Scimone A, Harvey R, Schofield P 2007 Altered motor activity, exploration, and anxiety in heterozygous neuregulin 1 mutant mice, implications in understanding schizophrenia. Genes Brain Behav 6:677–687 [DOI] [PubMed] [Google Scholar]
- Sandberg R, Yasuda R, Pankratz D, Carter T, Lockhart D, Barlow C 2000 Regional and strain-specific gene expression mapping in the adult mouse brain. Proc Natl Acad Sci USA 97:11043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupnik M, Ridgway E 1987 Thyroid hormone control of thyrotropin gene expression in rat anterior pituitary cells. Endocrinology 121:619–624 [DOI] [PubMed] [Google Scholar]
- McCreary A, Handley S 1999 The thyrotropin-releasing hormone analogue MK771 induces tic-like behaviours: the effects of dopamine D1 and D2 receptor antagonists. Eur J Pharmacol 369:1–9 [DOI] [PubMed] [Google Scholar]
- de Magalhaes-Nunes A, Badaue-Passos DJ, Ventura R, Guedes Dda SJ, Araujo J, Granadeiro P, Milanez-Barbosa H, da Costa-e-Sousa R, de Medeiros M, Antunes-Rodrigues J, Reis L 2007 Sertraline, a selective serotonin reuptake inhibitor, affects thirst, salt appetite and plasma levels of oxytocin and vasopressin in rats. Exp Physiol 92:913–922 [DOI] [PubMed] [Google Scholar]
- Jayawant S, Halpin S, Wallace S 2000 Menkes kinky hair disease: an unusual case. Eur J Paediatr Neurol 4:131–134 [DOI] [PubMed] [Google Scholar]
- Steveson TC, Ciccotosto GD, Ma XM, Mueller GP, Mains RE, Eipper BA 2003 Menkes protein contributes to the function of peptidylglycine α-hydroxylating monooxygenase. Endocrinology 144:188–200 [DOI] [PubMed] [Google Scholar]
- Sheela SR, Latha M, Lui P, Lem K, Kaler SG 2005 Copper-replacement treatment for symptomatic Menkes disease: ethical considerations. Clin Genet 68:278–283 [DOI] [PubMed] [Google Scholar]
- Turnlund JR 1998 Human whole-body copper metabolism. Am J Clin Nutr 67:960S–964S [DOI] [PubMed] [Google Scholar]
- Scott KC, Turnlund JR 1994 Compartmental model of copper metabolism in adult men. J Nutr Biochem 5:342–350 [Google Scholar]
- Scientific Committee on Food, European Commission on Health and Consumer Protection Directorate 2003 Opinion on the Scientific Committee on Food on the Tolerable Upper Level Intake of Copper. SCF/NUT/UPPLEV/57 Final [Google Scholar]
- Committee on Copper in Drinking Water: Board of Environmental Studies and Toxicology; Commission on Life Sciences; National Research Council 2000 Copper in drinking water. Washington, DC: National Academy Press [Google Scholar]
- Tan J, Burns D, Jones H 2006 Severe ataxia, myelopathy, and peripheral neuropathy due to acquired copper deficiency in a patient with history of gastrectomy. J Parent Enteral Nutr 30:446–450 [DOI] [PubMed] [Google Scholar]
- Linster C, Schaftingen E 2007 Vitamin C: biosynthesis, recycling, and degradation in mammals. FEBS J 274:1–22 [DOI] [PubMed] [Google Scholar]
- Ciccotosto GD, Schiller MR, Eipper BA, Mains RE 1999 Induction of integral membrane PAM expression in AtT-20 cells alters the storage and trafficking of POMC and PC1. J Cell Biol 144:459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlief M, Craig A, Gitlin J 2005 NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J Neurosci 251:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlief M, West T, Craig A, Holtzman D, Gitlin J 2006 Role of Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc Natl Acad Sci USA 103:14919–14924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Wong K, Horrigan F 2008 An extracellular Cu2+ binding site in the voltage sensor of BK and Shaker potassium channels. J Gen Physiol 131:483–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.