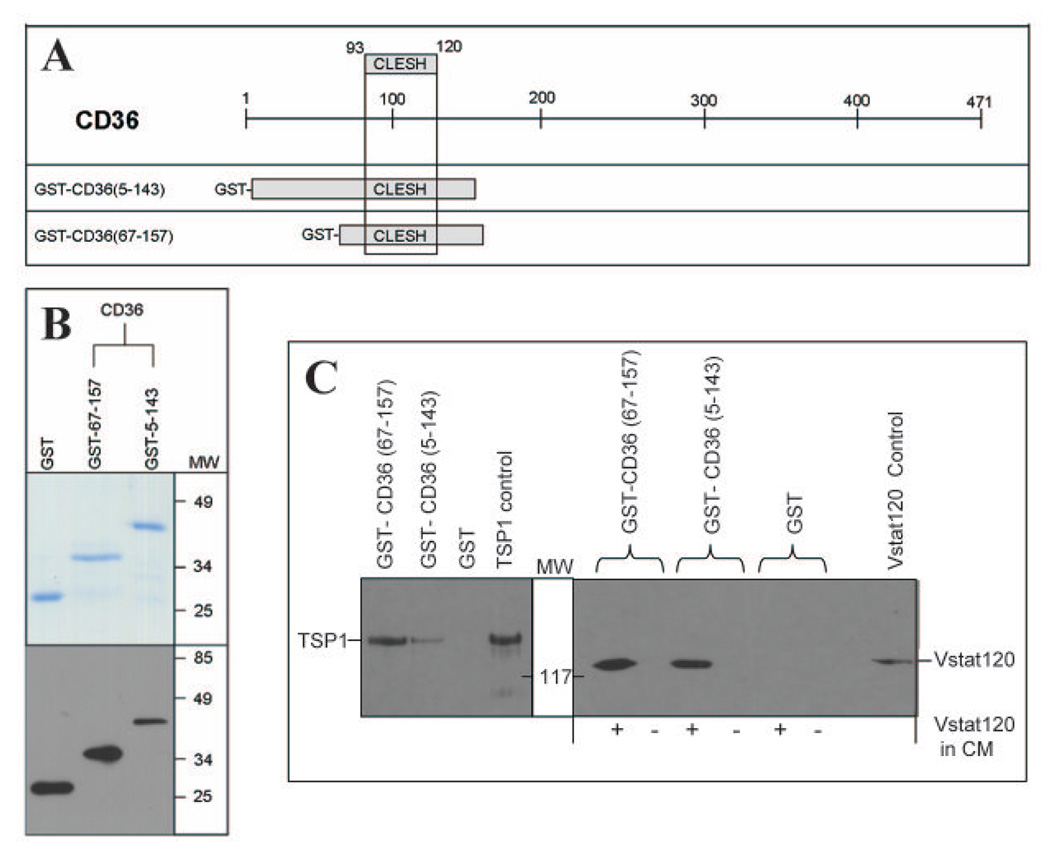
Figure 5. Vstat120 binds to the purified CLESH domain of CD36.
A: Schematic of CD36 structure with the CLESH domain. The two GST-CD36 constructs used (amino acids 5–143 and 67–157), both of which contain the CLESH domain (amino acids 93–120) are shown.
B. Top: Coomassie stained gel showing purified GST, and GST tagged recombinant proteins encoding for amino acids 67–157 and 5–143 of CD36. Proteins purified to near homogeneity and migrated at their predicted molecular weight. Bottom: Western blot analysis of each fusion protein probed with anti-GST monoclonal antibody (MAB3317 Chemicon International).
C. GST pull-down assay. GST alone or the two recombinant GST-CD36 peptides were bound to glutathione sepharose beads and CM from LN229 glioma cells stably expressing Vstat120 (+ lanes) or control cells (− lanes) was tested for protein interaction. A separate pull-down assay with CM from TSP1 expressing cells (LN229 clone C9) was used as a positive control. The bound proteins were eluted and analyzed for Vstat120 and TSP1 expression by western blot. Both the GST tagged CD36 containing recombinant peptides could pull down Vstat120 and TSP1 but not the purified GST. Positive control lanes are TCA precipitations of CM (collected serum-free after 96hrs) that express either Vstat120 or TSP1.