The mammary epithelium comprises two major cell types, basal and luminal. Basal cells directly interact with the extracellular matrix (ECM) and express higher levels of the ECM receptors, integrins, than luminal cells. We show that the deletion of β1 integrin from basal cells abolishes the regenerative potential of the mammary epithelium and affects mammary development. The mutant epithelium is characterised by an abnormal ductal branching pattern and aberrant morphogenesis in pregnancy, although at the end of gestation, the secretory alveoli develop from β1 integrin-positive progenitors. Lack of β1 integrin alters the orientation of the basal cell division axis, and in mutant epithelium, unlike control tissue, the progeny of β1 integrin-null basal cells, identified by a genetic marker, is found in the luminal compartment. These results, for the first time, reveal the essential role of the basal mammary epithelial cell-ECM interactions mediated by β1 integrins in the maintenance of the functional stem cell population, mammary morphogenesis and segregation of the two major mammary cell lineages.
The ability of the mammary epithelium to reconstitute the gland in serial transplants, strongly suggests that the adult mammary epithelium harbours a population of cells with a high regenerative potential — mammary stem cells1–3. Recently, populations enriched in stem cells have been isolated from adult mouse mammary tissue using surface markers, CD24, β1 or α6 integrin chains4,5. These populations have been found to be negative for steroid hormone receptors and consisted of cells that expressed basal markers6. Accordingly, our previous studies performed with derivatives of the mouse mammary cell line Comma D1 showed that morphogenetic potential was associated with the basal cell constituent of this heterogeneous cell population7,8. These results suggested that mammary stem cells might reside in the basal compartment of the mammary epithelium and express high amounts of integrins. However, the question of whether β1 integrins are important for the maintenance and function of this basal-type candidate stem cell population has not been addressed yet. Transgenic expression of a dominant-negative β1 integrin or ablation of the β1 integrin gene in mammary luminal cells in mice, demonstrated a role for β1 integrins in the control of mammary epithelium growth and differentiation but resulted in relatively mild phenotypes9–12. We therefore focused our study on the functions of major ECM receptors — β1 integrins — in basal epithelial cells.
The basal compartment of the adult mammary epithelium consists of differentiated myoepithelial cells that express markers of basal epithelial and smooth muscle cells, and includes a small population of cells expressing basal markers only13. Hereafter, we apply the term “basal” to both of these cell populations.
To delete β1 integrin from the basal cell layer of the mammary epithelium, mice harbouring floxed itgb1 alleles (itgb1F/F) were crossed with transgenic mice expressing Cre recombinase under control of the keratin 5 (K5)-promoter active in the basal cells of stratified and pseudostratified epithelia such as epidermis and mammary gland14–16. To prove that K5 promoter targets Cre expression to the basal layer of the mammary epithelium, basal and luminal mammary epithelial cells were isolated from K5-Cre transgenic mice using flow cytometry technique (Supplementary Information, Fig. S1). Evaluation of luminal (18) and basal (K14) marker expression by quantitative RT-PCR confirmed, that basal and luminal cells were found in the CD24-low/β1-high and CD24-high/β1-low fractions, respectively (Fig. 1a and Supplementary Information, Fig. S1). As expected, Cre expression was confined to the basal, CD24-low/β1-high, cell population (Fig. 1a).
Figure 1. Abnormal ductal morphogenesis in the mammary epithelium presenting conditional deletion of the β1 integrin gene in basal epithelial cells.
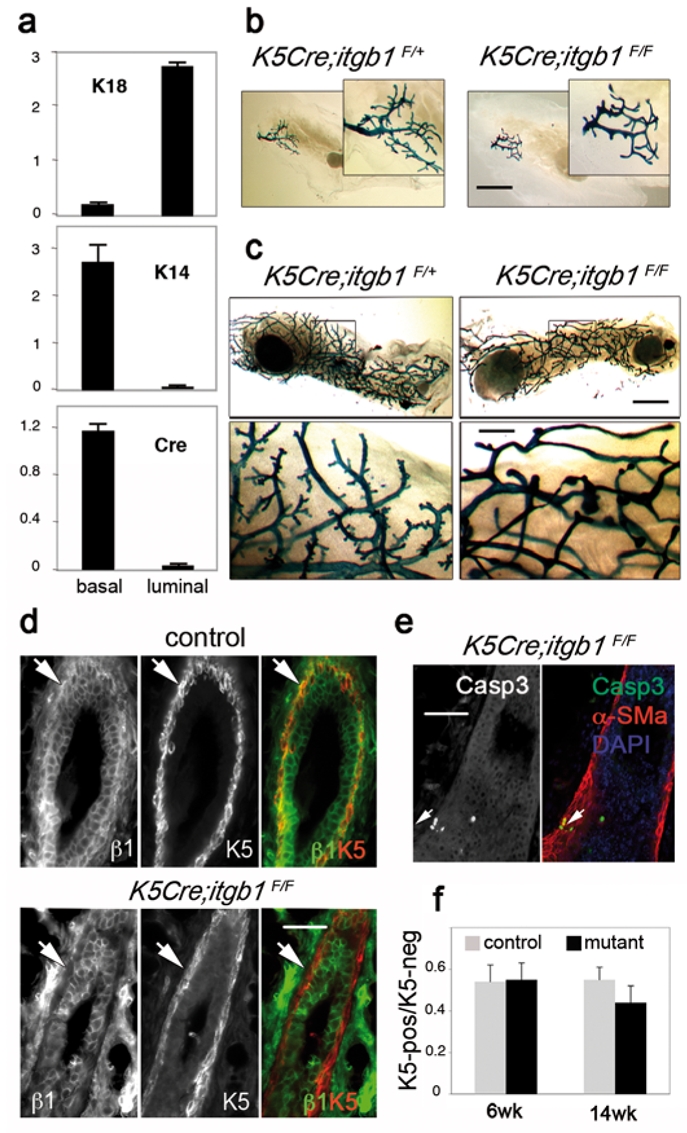
(a) Real time RT-PCR analysis of K18, K14 and Cre expression in basal and luminal cell populations isolated from 12-week-old virgin K5-Cre mouse mammary gland. Data are presented as means ± S.E.M. obtained in two independent experiments. (b) and (c) X-gal whole-mount staining. (b) Mammary rudiments from 3-week-old control (K5Cre;itgb1F/+) and mutant (K5Cre;itgb1F/F) mice; (c) outgrowths developed by control (K5Cre;itgb1F/+) and mutant epithelium in virgin recipient mouse. (d) and (e) Immunofluorescence staining of the sections through control and K5Cre;itgb1F/F outgrowths with anti-K5, anti-β1 integrin (d), anti-cleaved caspase 3 and α-SM-actin (α-SMa) (e) antibodies. Arrows in (d) indicate the basal cell layer. Arrows in (e) indicate cleaved caspase 3- and α-SM-actin-positive basal cells. DAPI served to stain nuclei. (f) Histograms showing ratio between K5-positive and K5-negative mammary epithelial cells in control and mutant outgrowths developed in virgin host. Data are presented as means ± S.E.M. of at least five mammary ducts from two animals in each case. Scale bars, 3 mm in (b) and (c), upper panel, 0.7 mm in (c), lower panel, and 55 μm in (d) and (e).
A lacZ-reporter gene introduced downstream to the floxed β1 integrin gene and expressed after Cre-mediated ablation was used to monitor β1 integrin gene deletion and to trace the progeny of the cells in which the gene has been deleted. Expression of the lacZ-reporter in the mammary epithelium of three-week-old mice confirmed the β1 integrin gene deletion (Fig. 1b).
The 3-week-old mutant mouse (K5Cre;itgb1F/F) mammary glands were similar in size to those of the wild type (not shown) and of K5Cre;itgb1F/+ animals, but had a slightly altered branching pattern (Fig. 1b). K5Cre;itgb1F/F mice develop a severe skin phenotype, and only rarely survive for more than five weeks17. Therefore, to proceed with the analysis of the mammary development, rudimentary glands from three- to four-week-old mutant and control littermates were grafted into the cleared fat pads of prepubertal nude Balb/c mice. For 92 grafted mice, 92 (100%) control and 79 (86%) mutant transplants produced outgrowths. Control outgrowths developed faster than mutant outgrowths. However, five to six weeks after transplantation, the grafted mutant epithelium, similar to control, occupied the entire mammary fat pad (Fig. 1c). In control transplants, the branching points were distributed regularly, and numerous short side branches typical of mature virgin mammary gland were present along the ducts. In contrast, mutant outgrowths were characterised by a largely disorganised general branching pattern and few side branches. β1 integrin was efficiently deleted from the basal cell layer (Fig. 1d), and the amount of β1 integrin-negative cells was estimated as, at least, 95% of total basal cell number. Although the majority of luminal cells remained positive for β1 integrin, small clusters of negative cells could be distinguished in the luminal cell layer (Supplementary Information, Fig. S2). Basal myoepithelial cells depleted of β1 integrin expressed the usual markers of this cell type and were able to proliferate (Fig. 1d and e and Supplementary Information, Fig. S3). Some basal cells (2.7 ± 0.5%) in mutant outgrowths stained positive for cleaved caspase 3, whereas no apoptotic cells were found in the basal layer of control glands (Fig. 1e and data not shown). However, within the observation period, the apoptosis did not result in any important decrease of basal cell number in K5Cre;itgb1F/F epithelium as the ratio between basal and luminal cells appeared to be similar in control and mutant outgrowths from 6-week-old mice and only slightly diminished in those from 14-week-old animals (Fig. 1f).
To assess the regenerative potential of K5Cre;itgb1F/F epithelium, small pieces of primary outgrowths were re-transplanted into the cleared fat pads of new recipient mice. In the secondary grafts, 16 out 18 (89%) mammary fat pads grafted with mutant epithelium either remained completely empty, or contained one to two small buds (Fig. 2a, upper right panel). The only two rudimentary outgrowths produced by mutant epithelium consisted of a few poorly branched ducts (Fig. 2a, lower right panel). Control epithelium, in most cases (16 out of 18, or 89%), produced large and elaborate outgrowths (Fig. 2a, left panels). These results demonstrate that the deletion of β1 integrin from basal cells abolished the regenerative potential of the mammary epithelium. Since in primary grafts, the lack of β1 integrin in the basal cells did not prevent proliferation, ductal growth and ramification, and only slightly increased apoptosis, the inability to re-establish a system of branching ducts in the secondary grafts strongly indicates a lack of functional stem cells in K5Cre;itgb1F/F epithelium.
Figure 2. Lack of functional stem cells in K5Cre;itgb1F/F epithelium.
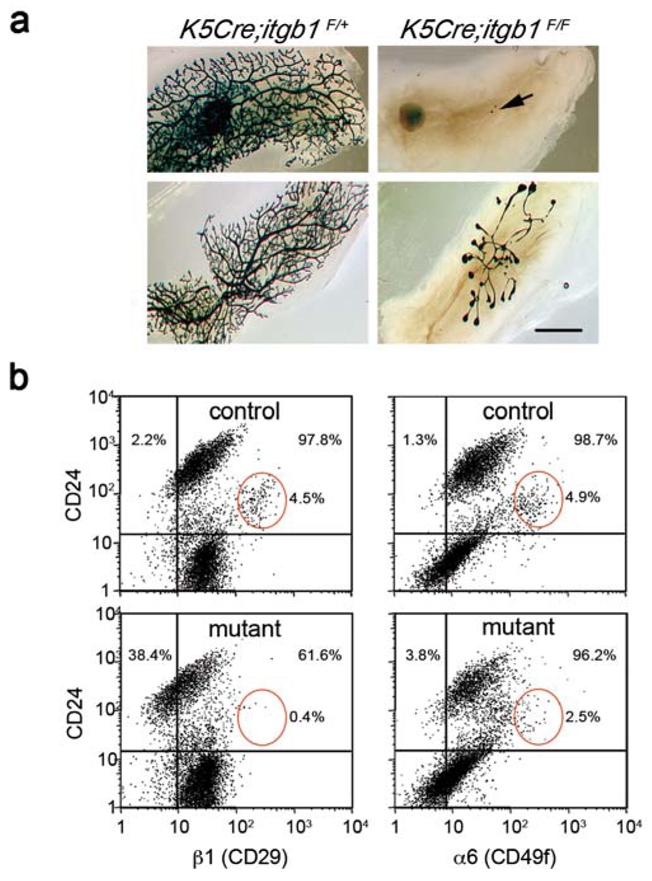
(a) Whole-mount X-gal staining of the secondary outgrowths produced by control K5Cre;itgb1F/+ and mutant K5Cre;itgb1F/F epithelium in the cleared fat pads of virgin recipient mice.
The outgrowths were analysed 10 weeks after transplantation. Arrow (upper panel) indicates small pieces of transplanted mutant epithelium that did not develop any secondary outgrowth. Lower panel shows the most developed mutant outgrowth and corresponding control. Scale bar, 3 mm.
(b) Flow cytometry analysis of mammary epithelial cells isolated from outgrowths developed by control (upper panels) and mutant (lower panels) epithelium in 12-week-old virgin recipient mice. Cells were stained for CD24 and β1 integrin (left) or CD24 and α6 integrin expression (right). Only CD45−CD31− cells were included in the analysis. Red ellipses show CD24-positive/β1-high and CD24-positive/α6-high cell populations. The percentages of β1- and α6-negative (left to vertical reference line) and positive (right to vertical reference line) cells were calculated for the CD24- positive population (above horizontal reference line) comprising mammary epithelial cells13.
Flow cytometry analyses supported this conclusion. As expected, mutant epithelium, in contrast to control, contained an important population of β1 integrin-negative cells (Fig. 2b, left panels). Mammary epithelial cells express α6β1 and α6β4 integrin dimers, therefore, the deletion of β1 integrin should not necessarily lead to a complete lack of α6 chain on the cell surface. Accordingly, the amount of α6-negative cells only slightly increased in K5Cre;itgb1F/F epithelium (Fig. 2b, right panels). In adult mouse mammary glands, stem cells have been associated with CD24-positive/β1-high or CD24-positive/α6-high cell populations4,5. In mutant epithelium, the amount of CD24-positive/β1-high cells dramatically decreased and that of CD24-positive/α6-high cells was two-fold lower than in control (Fig. 2b, red ellipses). Notably, as demonstrated by the secondary transplantation results, neither this residual CD24-positive/α6-high, nor any other cell population present in mutant epithelium, possessed a considerable regenerative potential.
To determine whether alveolar progenitors were affected by ablation of β1 integrin in basal cells, we analysed the mammary outgrowths developed in pregnant mice. Both, control and mutant epithelia responded to the stimulus of pregnancy by growth and changes in morphology (Fig. 3a). As expected, at 7.5 dpc, the lobuloalveolar development was clearly visible in the control epithelium, whereas in mutant outgrowths, only few alveolus-like structures developed before 14.5 dpc (Fig. 3a and b and Supplementary Information, Fig. S4). Instead, numerous buds reminiscent of small terminal end buds (TEBs) — bulbous structures found at the extremities of the growing ducts in virgin mouse glands — were formed in K5Cre;itgb1F/F outgrowths (Fig. 3b, d and e, right panels). Quantitative evaluation of milk gene expression confirmed that lobulo-alveolar development was retarded in mutant epithelium (Fig. 3C).
Figure 3. Perturbation of lobuloalveolar development in K5Cre;itgb1F/F mammary epithelium.
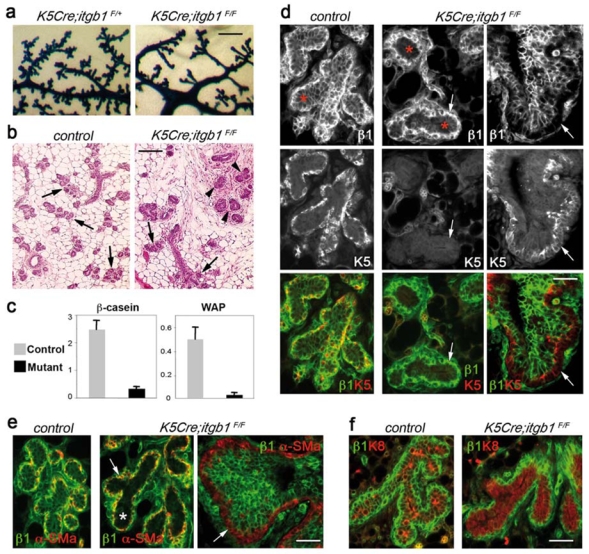
(a) Whole-mount X-gal staining of the K5Cre;itgb1F/+ and K5Cre;itgb1F/F outgrowths developed in 7.5-day-pregnant host. (b) H&E staining of the sections through control and K5Cre;itgb1F/F outgrowths from 14.5-day-pregnant host. Arrows and arrowheads indicate alveoli and TEB-like structures, respectively. (c) Quantitative RT-PCR analysis of β-casein and WAP expression in mammary glands from 13.5-daypregnant control and K5Cre;itgb1F/F mice. The values were normalised to K18 expression. Data are presented as means ± S.E.M. obtained in two independent experiments. (d-f) Double immunofluorescence staining of the sections through control and K5Cre;itgb1F/F outgrowths developed in 14.5- (d and e) and 7.5-day- day pregnant host (f) with anti-β1 integrin (d, e and f), anti-K5 (d), anti-α-SM-actin (e), anti-K8 (f) antibodies. Arrows in (d and e) indicate basal cell layer, asterisks show position of luminal layer. Scale bars, 0.77 mm in (a); 160 μm in (b), and 55 μm in (d–f).
Surprisingly, the basal cell layer of all alveolus-like structures and small ducts developed by mutant epithelium in pregnant recipient mice stained positive for β1 integrin, whereas many luminal cells appeared to be depleted of β1 integrin (Fig. 3d and e, central panels). Consistent with the presence of β1 integrin, basal cells in K5Cre;itgb1F/F alveoli were either completely negative, or weakly positive for K5 (Fig. 3d, central panels). Other mammary basal cell markers, such as α-SM-actin and p63, were expressed in the basal cell layer in control and mutant outgrowths (Fig. 3e and Supplementary Information, Fig. S5). Notably, in the TEB-like structures and in the ducts found in the mutant outgrowths, similar to the outgrowths formed in virgin mice, basal cells stained positive for K5 and were negative for β1 integrin (at least, 95% of total basal cell number). The multilayered luminal compartment comprised various proportions of β1 integrin-positive and -negative cells (Fig. 3d and e, right panels).
All luminal cells in mutant epithelium expressed luminal cell markers, such as K8 and ErbB2, and were negative for basal markers (Fig. 3d-f and Supplementary Information, Fig. S5). Luminal cells in mutant epithelium proliferated strongly but displayed higher levels of apoptosis than in control epithelium (Supplementary Information, Fig. S5). Thus, lobuloalveolar development in K5Cre;itgb1F/F epithelium was affected being characterised by (i) retarded alveologenesis, (ii) aberrant TEB-like structure formation, and (iii) unexpected presence of β1 integrin-negative cells in the luminal compartment of the epithelial bilayer.
The lack of β1 integrin expression in the luminal cells of K5Cre;itgb1F/F epithelium clearly indicated their origin from basal, K5-positive cells. Analysis of the expression of a genetic marker, lacZ, confirmed this conclusion. Most luminal cells in ducts and alveolus-like structures in mutant outgrowths analysed between 7.5 and 12.5 dpc stained blue with X-gal, with only seldom lacZ-negative cells detected (Fig. 4a, right panel). Thus, basal progenitor cells made a significant contribution to the luminal cell population of the mutant epithelium early in pregnancy. In contrast, in control K5Cre;itgb1F/+ epithelium, luminal cells were essentially LacZ-negative, showing that the vast majority of luminal cells did not originate from basal cells (Fig. 4a, left panel).
Figure 4. Altered orientation of the basal cell division axis in K5Cre;itgb1F/F mammary epithelium.
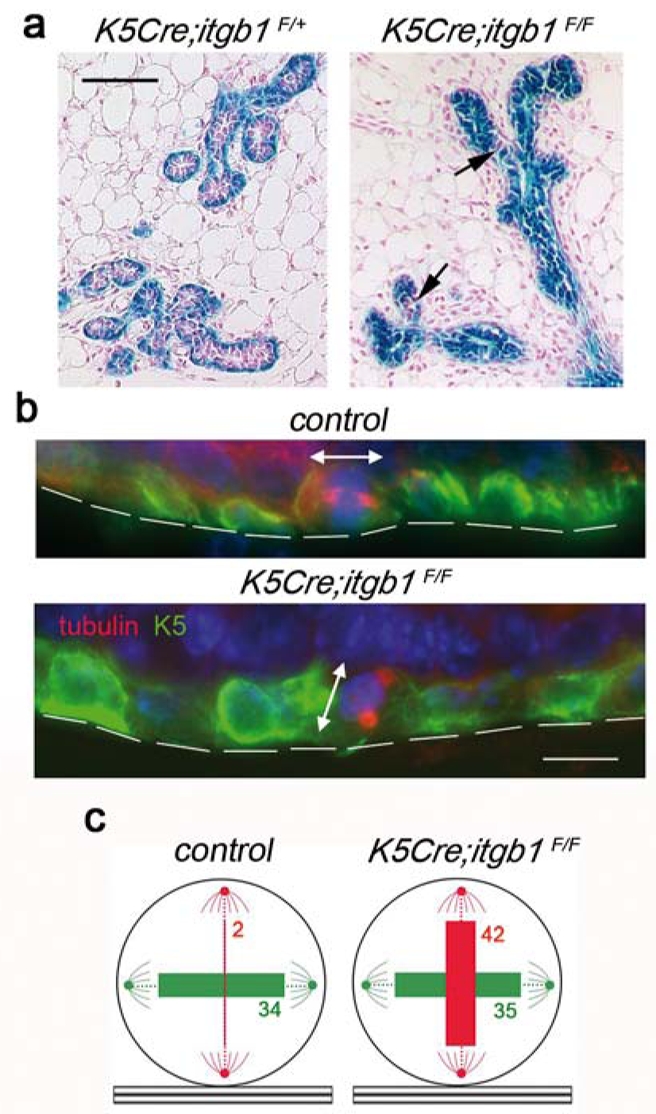
(a) Whole-mount X-gal staining of the K5Cre;itgb1F/+ and K5Cre;itgb1F/F outgrowths developed in 7.5-dpc host. Arrows show LacZ-negative (pink) cells. (b) Dividing basal cells in the ducts formed by control and K5Cre;itgb1F/F epithelium. Double immunofluorescence staining with anti-K5 and anti-β-tubulin antibodies. Basement membrane position is marked by discontinuous lines, double-headed arrows indicate division plane. (c) Position of ductal basal cell division plane in the mammary outgrowths developed in host mice at 7.5 dpc. The numbers in green and red correspond to the numbers of cells dividing parallel and perpendicular to the basement membrane, respectively. The thickness of colored bars is proportional to the cell number. Cell counts obtained for each of the four mice used for the analysis are presented in Supplementary Information, Table 1. Scale bars, 100 μm (a), and 75 μm (b).
Several recent studies demonstrated that cell-ECM interactions and in particular, those mediated by β1 integrins, play an important role in the organization and orientation of the mitotic spindle18–20. Quantitative evaluation of the basal cell division plane orientation in the ducts from the outgrowths developed in 7.5 dpc mice confirmed, that in accordance with the distribution of lacZ-positive cells, in control epithelium, in most cases, basal cells divided parallel to the basement membrane, whereas in mutant epithelium, orientation of the division plane was random, and numerous cells dividing perpendicular to the basement membrane were detected (Fig. 4b and c and Supplementary Information, Table 1). Thus, a lack of β1 integrins led to changes in the orientation of the basal cell division axis, strongly suggesting that in mutant epithelium, dividing basal cells produced basal and luminal cells instead of contributing to the basal cell population only.
At later stages of pregnancy (16.5 and 18.5 dpc), similar to control transplants, numerous well-developed, functionally differentiated alveoli were found in mutant outgrowths (Fig. 5a and Supplementary Information, Fig. S4 and Fig. S5). Similar to control tissue, alveolar cells in K5Cre;itgb1F/F epithelium, stained brightly with anti-β1 integrin antibody, whereas basal cells expressed K5 (Fig. 5c). X-gal staining of sections through 14.5, 16.5 and 18.5 dpc mutant outgrowths, showed that the amount of lacZ-negative cells dramatically increased in the ducts and TEB-like structures. Moreover, in the newly formed alveoli, luminal cells were lacZ-negative, whereas basal cells appeared to be either weakly positive, or negative. The aberrant TEB-like structures and some poorly developed alveoli persisted in K5Cre;itgb1F/F outgrowths (Fig. 5a, Supplementary Information, Fig. S4 and Fig. S5).
Figure 5. K5-negative progenitors give rise to functional alveoli late in pregnancy.
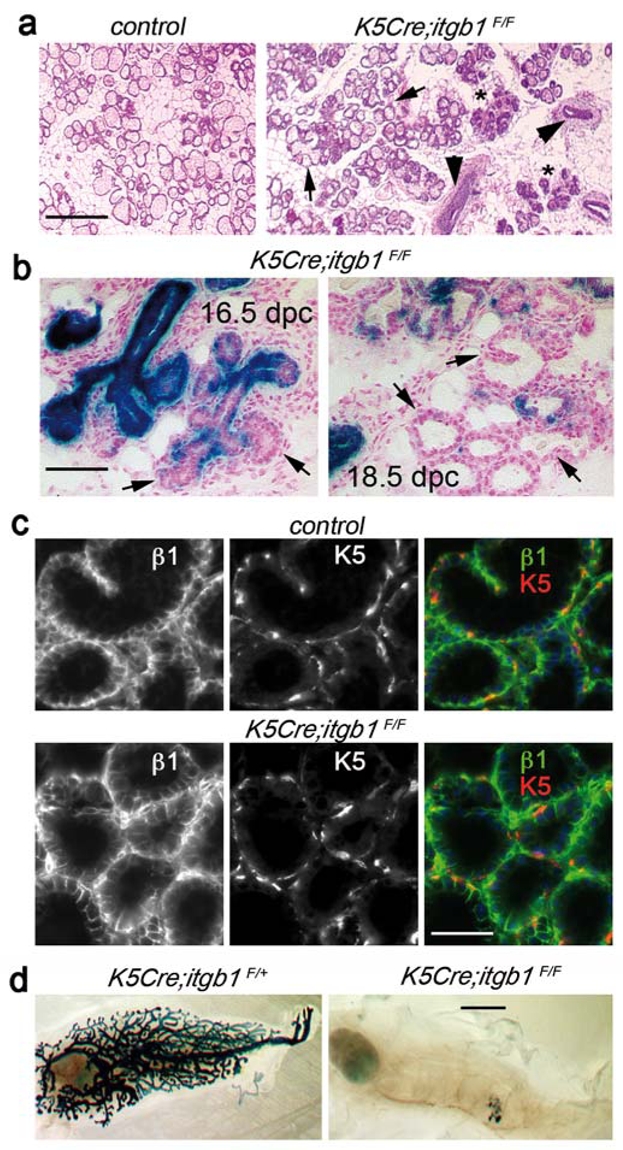
(a) H&E staining of the section through outgrowths developed by control and K5Cre;itgb1F/F epithelium in a 18.5-day-pregnant host. Arrows indicate well-developed alveoli in mutant epithelium; arrowheads and asterisks mark TEB-like structures and collapsed alveoli, respectively, persisting in K5Cre;itgb1F/F outgrowths. (b) X-gal staining of the sections through K5Cre;itgb1F/F outgrowths developed in 16.5- and 18.5-day-pregnant host. Arrows show newly formed alveoli consisting essentially of LacZ-negative cells. (c) Double immunofluorescence staining of the sections through control and K5Cre;itgb1F/F outgrowths developed in 18.5-daypregnant host with antibodies against β1 integrin and K5. (d) Whole-mount X-gal staining of secondary outgrowths resulting from re-transplantation of pieces of control (K5Cre;itgb1F/+) and mutant (K5Cre;itgb1F/F) epithelium developed in 15.5 daypregnant host mouse. In six out of seven grafted mice, control epithelium produced secondary outgrowths occupying the entire mammary fat pad, one outgrowth occupied approximately 30% of the fat pad. Mutant epithelium did not develop in five out of seven fat pads and produced two small outgrowths occupying approximately 10–15% of the fat pad. Scale bars, 0.3 mm in (a), 100 μm in (b), 75 μm in (c) and 3 mm in (d).
Thus, our data show that a population of progenitors activated late in pregnancy and able to give rise to the entire functional alveoli, including luminal and basal cells, was not affected by K5-promoter-driven deletion of β1 integrin from the mammary epithelium. Although it is difficult to completely exclude the possibility that a population of K5-positive progenitor cells might escape β1 integrin ablation, it seems likely that these alveoli originated from K5-negative progenitors. Other reports relying on colony-forming assays in culture, have suggested that alveoli might originate from luminal progenitors13,21,22. In addition, luminal-type progenitor cells activated during first pregnancy and able to contribute to the regeneration of the mammary gland after involution, were described earlier23,24. The non-uniform lacZ expression observed in the alveoli despite the high level of K5 expression in agreement with the results of immunofluorescence labelling, shows that β1 integrin was not yet deleted from the newly-formed alveolar basal cells (Fig. 5b and c).
Notably, the developmental potential of the progenitors activated late in pregnancy was limited to alveologenesis, as pieces of mutant epithelium, isolated from pregnant (15.5 dpc) host, were not able to produce ductal outgrowths in secondary transplants (Fig. 5d).
Cell polarisation and interaction with the basement membrane are essential for epithelial morphogenesis25,26. Thus, basal-type stem cells, due to their ability to produce basement membrane components and to properly organise the proximal ECM, may get an important advantage during the early events of fat pad repopulation, such as anchorage and establishment of baso-lateral polarity, required for the initiation of morphogenesis. Once the primitive ductal system has been formed, K5-negative progenitors with luminal characteristics should be able to reconstitute secretory alveoli.
Involvement of integrins in the control of multiple cellular functions suggests that β1 integrin gene ablation may lead to pleiotropic phenotypes. Consistently, primary mutant outgrowths developed slower than control tissue, presented altered ductal branching pattern and lobulo-alveolar development implicating β1 integrins in control of mammary morphogenesis, proliferative potential and survival of mammary epithelial cells. However, deletion of β1 integrins from basal cells did not impede the ductal growth and the differentiation of the two major mammary lineages, basal and luminal. A recent report concerning Drosophila gonad stem cells suggested that the lack of β1 integrin might alter ECM organisation and damage the stem cell niche27. In contrast to this system, our knowledge concerning mammary progenitor/stem cell identity, location and, in particular, the mammary stem cell niche, is limited. Further investigations are required to precisely characterise the mammary stem cell immediate environment and to determine how it contributes to the control of the mammary stem cell renewal and functioning. Essentially, the results of this study reveal a central role of basal cell interactions with ECM mediated by β1 integrins in the maintenance of mammary stem cell population.
Methods
Mouse strains
All animal experiments were conducted according to the French veterinary guidelines and those formulated by the Council of Europe for experimental animal use (L358-86/609EEC). Transgenic mice expressing the Cre-recombinase under control of the keratin-5 promoter (K5-cre mouse line) were kindly provided by Dr. J. Jorcano15. The generation of mice carrying conditional β1 integrin alleles has been described elsewhere17,28. All mice were bred in 129SV/C57BL6 genetic background. Wild type, itgb1F/+, itgb1F/F, and, when indicated, K5Cre;itgb1F/+ mice were used as control. itgb1F/+, itgb1F/F and K5Cre;itgb1F/+ mice developed normally, their mammary glands were found to be similar to those of wild type animals, and the females were able to feed normal size litters.
Transplantation of mammary epithelium
Pieces of approximately 1 mm3 dissected from the mammary fat pad region adjacent to primary duct and containing mammary rudiment clearly visible with the dissection microscope (donor epithelium) were implanted into the inguinal number four fat pads of 3-week-old nude balb/c females cleared of endogenous epithelium, as described elsewhere29. Pieces of tissue removed from the recipient fat pad were stained with Carmine to control for the elimination of endogenous epithelium. In each case, mutant (K5Cre;itgb1F/F) and control (wild type, itgb1F/+, itgb1F/F, and, when indicated, K5Cre;itgb1F/+) epithelia were grafted into the contralateral fat pads of the same recipient mouse. The outgrowths produced were analyzed by dissecting recipient fat pads three to 15 weeks after transplantation. For secondary transplantation, small pieces of the primary outgrowths developed in 12- to 14-week-old virgin or pregnant hosts were dissected and re-grafted into new recipient mice following the same protocol.
X-gal staining
For whole-mount X-gal staining, mammary glands were fixed by incubation in 2.5% paraformaldehyde in PBS, pH 7.5, for 1 h at 4°C, and stained overnight at 30°C (Biology of the Mammary Gland, http://mammary.nih.gov). Alternatively, cryosections (10–15 μm) were cut from mammary gland pieces embedded in Tissue-Tek (Miles Diagnostic Division, Elkhart, IN) and frozen in isopentane cooled by liquid nitrogen. For X-gal staining, the cryosections were thawed at room temperature for 30 min, fixed for 15 min in 2% formaldehyde, 0.2% gluteraldehyde, 0.02% NP-40 in PBS, washed with PBS and incubated with X-gal staining solution (0.025% X-gal, 3 mM K3Fe(CN)6, 3 mM K4Fe(CN)6, 1.5 mM MgCl2, 15 mM NaCl, 40 mM Hepes, pH 8) overnight at 30 °C. Sections were then post-fixed with 4% formaldehyde, and counterstained with Nuclear Fast Red.
BrdU-incorporation test, histology and immunostaining
Mice were intraperitonealy injected with bromodeoxyuridine (BrdU), 0.25 mg/g of body weight, 2 h before tissue collection. Dissected mammary fat pads were spread onto a glass slide, fixed in MethaCarn, and embedded in paraffin. Sections (7 μm) were cut and deparaffinised before hematoxylin/eosin or immunofluorescence staining, as described elsewhere16. Sections were incubated overnight at 4°C with primary antibodies, 1 hour at room temperature with secondary antibodies and 3 min with DAPI.
The following primary antibodies were used for immunostaining: rabbit polyclonal anti-laminin (Sigma-Aldrich, 1/100), anti-keratin 5 (Covance, 1/2000), anti-Stat5 (Santa Cruz Biotechnology, 1/500), anti-ErbB2 (Santa Cruz Biotechnology, 1/50), anti-cleaved caspase 3 (R&D Systems, 1/100), anti-pankeratin (DAKO, 1/100); rabbit monoclonal anti-Ki67 (clone SP 6, Neo Markers, 1/200); rat monoclonal anti-β1 integrin (clone MB 1.2, Chemicon, 1/200); mouse monoclonal anti-BrdU (clone 3D4, BD Pharmingen, 1/100), anti keratin 8 (clone Ks 8.7, Progen Biotechnik, 1/100), anti-α-SM-actin (1A4, Sigma-Aldrich, 1/100), anti-p63 (clone 4A4, BD Pharmingen, 1/100), anti-β-tubulin (clone SAP.4G5, Sigma-Aldrich, 1/100).
Antigen retrieval was performed by boiling the slides in 10 mM citrate buffer pH 6, for 10 min in microwave for BrdU, cleaved caspase 3, p63, Ki67, β-tubulin and ErbB2 detection. All secondary antibodies were Alexafluor-conjugated (1/1000, Molecular Probes).
Preparation of the mammary epithelial cells and flow cytometry
To isolate mammary epithelial cells, mammary fat pads were mechanically dissociated with scissors and scalpel and digested for 90 min at 37°C in CO2-independent medium (Invitrogen) supplemented with 5% foetal bovine serum, 3 mg ml−1 collagenase (Roche Diagnostics) and 100 U ml−1 hyaluronidase (Sigma). The resultant suspension was sequentially resuspended in 0.25% trypsin-EDTA for 1 min, and then 5 min in 5 mg ml−1 dispase (Roche Diagnostics) plus 0.1 mg ml−1 DNase I (Sigma) followed by filtration trough a 40-μm mesh. Red blood cells were lysed in NH4Cl.
To separate basal and luminal cells, mammary epithelial cells isolated from the inguinal glands of five 12-week-old virgin K5-Cre mice were pooled, stained with anti-CD24-PE (clone M1/69; BD Pharmingen), anti-CD29-FITC (clone OXM178; Chemicon), anti-CD45-APC (clone 30-F11; Biolegend) and anti-CD31-APC (clone MEC13.3; Biolegend) antibodies as described elsewhere4,6. CD24-low/β1-high (basal) and CD24-high/β1-low (luminal) cells were purified using FACSvantage (Becton Dickinson) and used to isolate RNA to quantitatively evaluate gene expression. CD45- and CD31-positive stromal cells were excluded from the flow cytometry analysis. Conjugated isotype-matching IgGs were used as negative controls.
Analysis of cell populations present in mammary epithelial outgrowths was performed with the cells isolated from the mammary fat pads from 12-week-old virgin mice transplanted with control or mutant epithelium. Cells stained with anti- CD24-PE, anti-CD49f-FITC (clone GoH3; BD Pharmingen), anti-CD29-FITC, anti-CD45-APC and anti-CD31-APC antibodies were analysed using a FACScalibur (Becton Dickinson) as described above. Two independent experiments were performed, each with three control and three mutant outgrowths pooled to isolate mammary epithelial cells. Similar results were obtained in both cases; data obtained in one of the experiments are presented.
Quantitative evaluation of β1 integrin deletion from basal cells
β1 integrinnegative and positive basal cells revealed by immunolabelling for K5 were counted in mammary ducts from the outgrowths developed by mutant epithelium in virgin and pregnant host. In each case, five different outgrowths were included into analysis, and, at least, 2000 of K5-positive cells per outgrowth were counted. The data are presented as mean ± S.E.M.
Quantitative RT-PCR
RNAs were isolated from mammary epithelial cells obtained by cell sorting or from transplanted mammary fat pads using RNeasy kit (Quiagen Gmbh). RNA (0.5–1 μg) was treated with MMLV H(−) Point reverse transcriptase (Promega), and quantitative PCR was performed by monitoring in real time the increase in fluorescence of the SYBR Green dye on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). To evaluate β-casein and WAP, p63 and P-cadherin expression, RNAs were isolated from the outgrowths developed in three 13.5 day-pregnant and three 14-week-old virgin hosts, respectively. The sequences of the primers used is provided in the Supplementary Information.
Quantitative evaluation of the orientation of the basal cell division plane
Cells in late metaphase, anaphase and early telophase were used for quantification. In the epithelial outgrowths isolated from four transplanted nude mice at 7.5 dpc, we counted 36 and 77 dividing basal cells for control and mutant epithelium, respectively. Division planes positioned at 60 to 90° to the basement membrane were classed as perpendicular, those that were oriented at 0 to 30° were classed as parallel. Fisher and Chi-squared tests were used for statistical evaluation.
Supplementary Material
Acknowledgments
We are particularly grateful to Dr. I. Grandjean, and the personnel of the animal facilities at Institut Curie for taking care of the mice and to Z. Maciorowski and A. Viguier for assistance with FACS analyses. We would also like to thank Dr. J.L. Jorcano for providing the K5-cre mice, Drs. M. Bissell, C. Brakebusch and M. Moumen for valuable discussions, Dr. A.-M. Valles for comments on the manuscript and M. Denoyelle for technical assistance. This work was supported by the Association pour la Recherche contre le Cancer (ARC 3295 and 3771) and La Ligue Nationale Contre le Cancer (Equipe Labelisée 2006). I.T. was supported by a grant from Fondation pour la Recherche Médicale (FRM). MMF and MAD are Chargés de Recherche, and MAG is Directeur de Recherche at the Institut National de la Santé et de la Recherche Médicale (INSERM).
References
- 1.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 2.Visvader JE, Lindeman GJ. Mammary stem cells and mammopoiesis. Cancer Res. 2006;66:9798–9801. doi: 10.1158/0008-5472.CAN-06-2254. [DOI] [PubMed] [Google Scholar]
- 3.Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer. 2003;3:832–844. doi: 10.1038/nrc1212. [DOI] [PubMed] [Google Scholar]
- 4.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 5.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 6.Asselin-Labat ML, et al. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98:1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- 7.Deugnier MA, et al. EGF controls the in vivo developmental potential of a mammary epithelial cell line possessing progenitor properties. J Cell Biol. 2002;159:453–463. doi: 10.1083/jcb.200207138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deugnier MA, et al. Isolation of mouse mammary epithelial progenitor cells with basal characteristics from the Comma-Dbeta cell line. Dev Biol. 2006;293:414–425. doi: 10.1016/j.ydbio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Perturbation of beta1-integrin function alters the development of murine mammary gland. Embo J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White DE, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Li N, et al. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. Embo J. 2005;24:1942–1953. doi: 10.1038/sj.emboj.7600674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naylor MJ, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sleeman KE, et al. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gusterson BA, Ross DT, Heath VJ, Stein T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005;7:143–148. doi: 10.1186/bcr1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez A, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- 16.Teuliere J, et al. Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005;132:267–277. doi: 10.1242/dev.01583. [DOI] [PubMed] [Google Scholar]
- 17.Brakebusch C, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. Embo J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reverte CG, Benware A, Jones CW, LaFlamme SE. Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J Cell Biol. 2006;174:491–497. doi: 10.1083/jcb.200603069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Miñán A, Martín-Bermudo MD, González-Reyes A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicularepithelium monolayer. Curr Biol. 2007;17:683–688. doi: 10.1016/j.cub.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 21.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asselin-Labat ML, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 23.Wagner KU, et al. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377– 1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- 24.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Gibson MC, Perrimon N. Apicobasal polarization: epithelial form and function. Curr Opin Cell Biol. 2003;15:747–752. doi: 10.1016/j.ceb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. doi: 10.1038/ncb1660. Published on line 4 novembre 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potocnik AJ, Brakebusch C, Fassler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 29.Deome KB, Faulkin LJ, Jr, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


