Abstract
Conditions are described for the Rh-catalyzed formation of highly-functionalized dihydro-and tetrahydrofuran products via three-component reactions of aldehydes, α-alkyl-α-diazoesters, and dipolarophiles. The alkyl-substituted carbonyl ylides that are generated in this fashion are highly reactive in cycloaddition reactions, and display a scope of reactivity that is much broader than three-component reactions of carbonyl ylides derived from ethyl diazoacetate or α-aryl-α-diazoesters. The reactions of alkyl-substituted carbonyl ylides proceed with high regioselectivity and diastereoselectivity that are rationalized by an asynchronous, endo-selective transition state.
INTRODUCTION
Dipolar cycloadditions are powerful reactions that rapidly build structurally complex heterocycles, and the multicomponent nature of dipolar cycloaddition reactions have been used to great effect in discovery chemistry.1 Metal-catalyzed cycloadditions involving carbonyl ylides can generate stereochemically complex products from three simple starting materials.2 The scope of such cycloadditions is broad when carbonyl ylides are formed by intramolecular processes.2 In contrast, analogous three-component reactions involving aldehydes, diazo compounds and dipolarophiles had been relatively limited in terms of selectivity and substrate scope.3 Because of competing dioxolane formation,3a,4,5 the scope is generally limited to highly activated dipolarophiles (e.g. maleate), and mixtures of regio- and stereoisomers are often obtained.
In an elegant study, Jamison investigated the Rh-catalyzed reactions of carbonyl ylides derived from trimethylsilyldiazomethane and dicobalt-hexacarbonyl complexes of propargyl aldehydes.3e The diastereoselectivity and the substrate scope of the Jamison system was high. However, regioselectivity was an issue for many of those transformations.3e Muthusamy described Rh-catalyzed reactions of cyclic diazoamides, aldehydes and dimethyl acetylenedicarboxylate that proceed with high diastereoselectivity, but regioselectivity was low when ethyl acrylate was substituted as the dipolarophile.3f Nair demonstrated that reactions of diazomalonate, aromatic aldehydes, and β-nitrostyrene proceed with high regio- and stereocontrol.3g Despite these considerable advances, there was still not a general three-component method for combining aldehydes, diazocompounds and dipolarophiles with broad scope and control over regio- and diastereoselectivity. Accordingly, we sought to develop three-component reactions that would parallel the large scope of cycloadditions with cyclic carbonyl ylides.2
We recently demonstrated that carbonyl ylides of structure 1 could be generated from α-alkyl-α–diazoesters at low temperature with catalytic Rh2Piv4.4g Such ylides were not previously accessible due to the propensity of the precursor Rh-carbenoids to undergo β–hydride elimination.6 Ylides 1 were shown to react with excess aldehyde at −78 °C to produce dioxolanes in high diastereoselectivity. We reasoned that the alkyl substituents of 1 destabilize the formal negative charge on the ylide, and consequently that ylides 1 should display enhanced reactivity toward exogeneous dipolarophiles (Scheme 1). Herein, it is demonstrated that Rh2Piv4 catalyzed, three-component reactions of aldehydes, α-alkyl diazoesters and dipolarophiles give a diverse range of tetrahydrofuran (2) or dihydrofuran products (Scheme 1) with an unusual reaction scope and high selectivity. The high regio- and diastereoselectivity is rationalized by the asynchronous, endo-transition state displayed in Scheme 1.
Scheme 1.
Cycloadditions of alkyl-substituted carbonyl ylides
RESULTS AND DISCUSSION
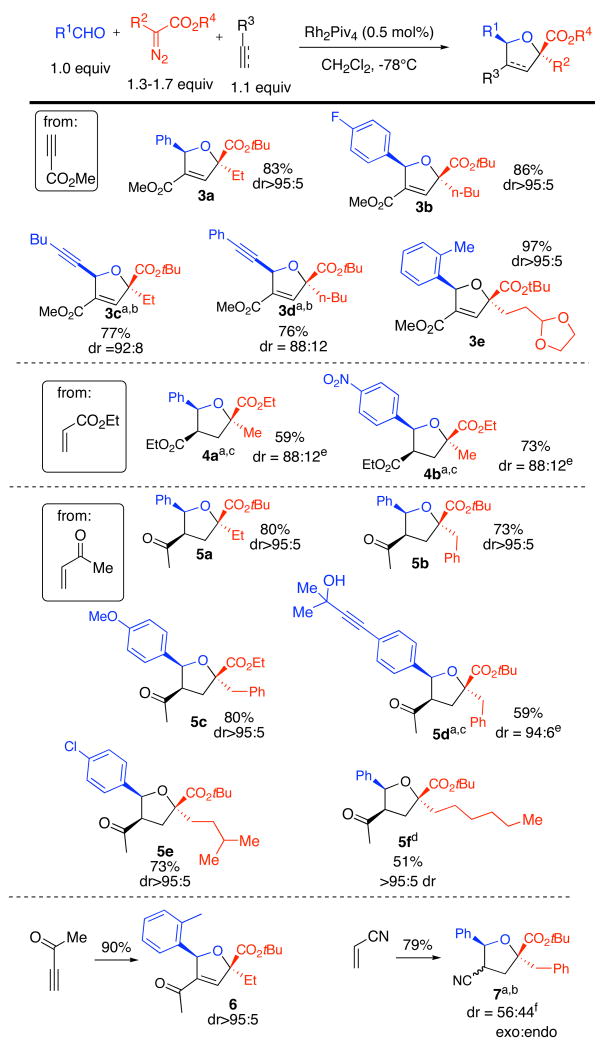
Dirhodium tetrapivalate has previously been shown4g to be an efficient catalyst for the formation of carbonyl ylides from α-alkyl–α-diazoesters and benzaldehyde derivatives. It was found that methyl vinyl ketone was able to intercept the carbonyl ylide generated from benzaldehyde and tert-butyl 2-diazohydrocinnamate in the presence of Rh2Piv4 at −78 °C to form the functionalized tetrahydrofuran product with high regio- and diastereoselectivity. Following an optimization study (Table 1), it was found that yields were the highest when benzaldehyde was used as the limiting reagent with a small excess of tert-butyl 2-diazohydrocinnamate and methyl vinyl ketone. However, when these conditions were applied to the analogous system with tert-butyl 2-diazopropanoate, the reaction did not go to completion and yields were lower. For this system, as well as for other in which the diazoester is substituted with a simple alkyl chain (R = Me, Bu), it was necessary for 1.7 equivalents of diazo compound to be used to push the reaction to near-completion. Hashimoto’s dirhodium tetrakis[N-phthaloyl-(S)-tert-leucinate catalyst7 was tested for asymmetric induction in the reaction of p-anisaldehyde, tert-butyl 2-diazohydrocinnamate, and methyl vinyl ketone, however the product that was obtained was racemic. Rh2(OAc)4 and Cu(acac)4 were also examined as catalysts in the reaction between methyl vinyl ketone, benzaldehyde and tert-butyl 2-diazohydrocinnamate at −78 °C. β-Hydride elimination predominated with Rh2(OAc)4, and tetrahydrofuran 5 (R = Bn) was obtained in less than 20% yield. The tetrahydrofuran products were not formed at all in the reaction with Cu(acac)2, which lead to products that we have not yet been able to identify.
Table 1.
Optimization Study

|
NMR yield
Reaction was carried out at room temperature
The conditions from Table 1 were applied to a variety of alkynes and alkenes with a single activating group to give products 3–7 (Scheme 2). Successful reactivity was observed for α-diazopropionate, α-diazobutanoate, α-diazooctanoate, α-diazo-5-methylhexanoate, α-diazo-4-[1,3]dioxolan-2-yl-butanoate, and α-diazo-hydrocinnamate with a range of aromatic aldehydes or with propargyl aldehydes. Dipolarophiles include methyl propiolate, ethyl acrylate, methyl vinyl ketone, acrylonitrile and propargyl methyl ketone. Prior use of such dipolarophiles in three-component reactions with carbonyl ylides had been limited, and in those instances ≤3:1 regioselectivity was observed.3e,f The 3-component reactions in Scheme 2 generally proceeded with excellent selectivity: high selectivity (>88:12) was observed for 11 of the 12 examples in Scheme 2, and only one isomer (>95:5) was observed in 7 cases. The exceptional case was the reaction of acrylonitrile, benzaldehyde and tert-butyl 2-diazohydrocinnamate, which proceeded to give 7 with high regioselectivity but with poor stereoselectivity. For the formation of 5f, cyclopentane formation via intramolecular C–H activation was a competing side reaction, but the use of excess benzaldehyde (4.0 equiv) and dipolarophile (4.0 equiv) did give 5f in acceptable yield (51%). By contrast, intramolecular C–H activation was not a competing side reaction for the formation of 5e from tert-butyl α-diazo-5-methylhexanoate.
Scheme 2. Three-component coupling reactions of dipolarophiles with a single activating group.
aTwo diastereomers were detected upon analysis of the 1H NMR spectrum of the crude. Other isomeric materials were not detected. bYield of both diastereomers. cYield of diastereomer shown. dThe reaction was carried out with 1.0 equiv of t-butyl-α-diazooctanoate, 4.0 equiv of benzaldehyde and 4.0 equiv. of methyl vinyl ketone. eThe relative stereochemistry of the minor isomer was not determined. fStereochemical assignments were made on the basis of 1H NMR analysis (see Supporting information).
More highly substituted dipolarophiles also combine efficiently with alkyl substituted carbonyl ylides to give products 8–17, as shown in Scheme 3. Of the dipolarophiles in Scheme 3, only maleic anhydride and dimethyl acetylenedicarboxylate have been utlized previously2b,2d,3b in 3-component reactions involving carbonyl ylides. Unsymmetrical dipolarophiles also react efficiently with alkyl-substituted carbonyl ylides: the reactions of cyclohexenone, methyl methacrylate, and dimethyl 2-ethylidenemalonate proceed with high regioselectivity to give 8, 11 and 17, respectively. Diethyl azodicarboxylate successfully leads to the tetrahydrooxa-3,4-diazole 10. Strain can also be used to activate dipolarophiles:3e,8 prochiral cyclopropene 13 and chiral cyclopropene 15 react efficiently to give 14 and 16, respectively. Excellent regio- and diastereoselectivity (>95:5) was observed in all cases in Scheme 3 except for the reaction of methyl methacrylate, which gave 11 with 1:1 diastereoselectivity, and the reaction of maleic anhydride which gave 9b with 91:9 diastereoslectivity. A variety of functional groups are tolerated by the reactions in Schemes 2 and 3, including esters, alkynes, nitriles, oxazolidinones, alcohols, nitro-groups, and methoxy groups.9 As in the formation of 5f, cyclopentane formation via intramolecular C–H activation was a competing side reaction for the formation of 12b. However, the use of excess 4-fluorobenzaldehyde (4.0 equiv) and dipolarophile (4.0 equiv) gave 12b in good yield (63%).
Scheme 3. Three-component coupling reactions of dipolarophiles with multiple substitutents.
Diastereomer ratios were determined by analysis of the 1H NMR spectra of the crude: no other isomeric materials were observed. aIsolated yield of diastereomer shown. bIsolated yield of both diastereomers. cThe reaction was carried out with 1.0 equiv of t-butyl-α-diazooctanoate, 4.0 equiv of 4-fluorobenzaldehyde and 4.0 equiv. of dimethyl acetylenedicarboxylate. dStereochemical assignments were made on the basis of 1H NMR analysis (see Supporting information).
The reactions in scheme 2 and scheme 3 are subject to some limitations. Attempts to use 1-hexene, phenyl acetylene, norbornene, and vinyl trimethylsilane were unsuccessful, and led primarily to dioxolane products. An attempt to utilize tert-butyl α-diazoisovalerate led only to β–hydride elimination. Reactions with dimethyl maleate, ethyl-trans-crotonate, 3-pentyn-2-one, and acrolein gave the desired products in very low yield (≤ 10%). When alkyl aldehydes were used (propionaldehyde and pivaldehyde), only β-hydride elimination was observed. An attempt to utilize tert-butyl α-diazo-α-cyclohexylacetate led only to β–hydride elimination.
The generation and rapid reactivity of alkyl-substituted carbonyl ylides at low temperature is key to selective reactivity. At higher temperatures, the selectivity and yields are poor. When tert-butyl 2-diazohydrocinnamate is reacted with benzaldehyde and methyl vinyl ketone at rt, the tetrahydrofuran product is formed in only 16% yield, while the amount of β-hydride elimination (68% based on the diazo compound) is increased. Analogous reactions of ethyl diazoacetate or methyl phenyldiazoacetate with benzaldehyde and ethyl acrylate are less successful at −78 °C or at rt: The reaction with ethyldiazoacetate forms primarily diethyl maleate and diethyl fumarate at −78 °C or at rt. The reaction with methyl phenyldiazoacetate formed the desried product in low yield (31% based on 1H NMR analysis) with competing epoxide10 formation (18%); the remainder of the mass balance was unreacted aldehyde (16%) and a complex mixture of products.
To demonstrate that the cycloaddition was complete at −78 °C, the reaction of benzaldehyde, tert-butyl 2-diazohexanoate, and methyl propiolate was carried out according to the general procedure. Following the addition of the diazo compound, the reaction was allowed to stir at −78 °C for five minutes; then ethyl propiolate was added (1.1 equiv) and the reaction was allowed to warm up to room temperature. Analysis of the crude 1H NMR spectrum revealed that cycloaddition had only occurred with methyl propiolate. A control experiment was run in which both methyl propiolate (1.1 equiv) and ethyl propiolate (1.1 equiv) were added prior to diazo addition. Crude 1H NMR analysis revealed a ~1:1 mixture of both cycloadducts.
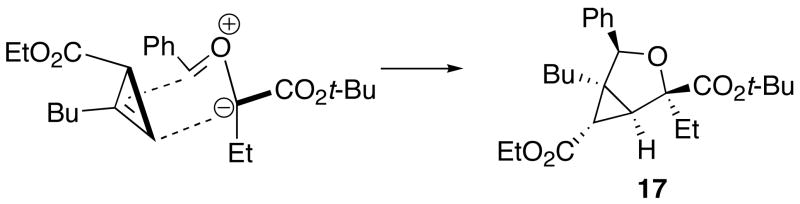
Regio- and stereochemical assignments were made on the basis of X-ray crystal structures for compounds 5b, 9a, and 14; NOE experiments on 17 and on both diastereomers of compound 3c; and chemical shift anisotropy analysis of the 1H NMR spectra for all compounds. In the majority of the cases studied, the major product arises from an endo approach of the dipolarophile to the ylide conformer A (Figure 2). An exceptional case was compound 17 derived from dimethyl 2-ethylidenemalonate in which the major product was formed by exo approach to the conformer A. Exo/endo isomers were also observed for 7 and 11, in which the dipolarophiles were acrylonitrile and methyl methacrylate, respectively. Products arising from cycloaddition with the minor ylide conformer, conformer B, were observed for 3c and 3d where propargyl aldehydes were employed. The minor isomers of 4a, 4b, 5d and 9b were not definitively assigned, but in analogy to our prior observations in dioxolane formation,4g we assume that they also arise from endo approach of the dipolarophile to the minor ylide conformer B.
Figure 2.
Proposed model to explain diastereoselectivity
When cyclopropenes 13 and 15 are used as dipolarophiles, the cyclopropene controls the endo-selectivity of the cycloadditions to provide the cycloaddition adducts 14 and 16 as single diastereomers. The sense of diastereoselectivity is in accord with that observed by Molchanov and coworkers in reactions between cyclic carbonyl ylides and substituted cyclopropenes.8 With the trisubstituted cyclopropene 15, the regioselectivity of dipolar cycloaddition is apparently controlled by steric considerations: the preferred regioisomer is derived from the transition state where the less substituted end of the cyclopropene is aligned with the more substituted end of the carbonyl ylide, as shown in Figure 3.
Figure 3.
Proposed model for the formation of 17
CONCLUSIONS
In summary, general conditions are described for the Rh-catalyzed formation of highly-functionalized dihydro- and tetrahydrofuran via three-component reactions of aldehydes, α-alkyl-α-diazoesters, and dipolarophiles with selectivity over dioxolane formation. Alkyl substituted carbonyl ylides are highly reactive in such transformations, and the scope of reactivity is broad relative to analogous carbonyl ylides derived from ethyl diazoacetate or α-aryl-α-diazoesters. Products are formed in good yields and with excellent regio- and diastereoselectivity when the reactions are carried out at − 78 °C in the presence of catalytic Rh2Piv4. A model invoking an asynchronous, endo transition state is proposed to explain the nature of diastereoselectivity.
EXPERIMENTAL SECTION
Representative procedure for three component dipolar cycloaddition reactions: synthesis of 4-acetyl-2α-ethyl-5β-phenyl-tetrahydrofuran-2β-carboxylic acid ethyl ester (5a)
In a flame-dried round bottomed flask, Rh2Piv4 (1.5 mg, 0.002 mmol), benzaldehyde (54 mg, 0.51 mmol), and methyl vinyl ketone (39 mg, 0.56 mmol) were dissolved in anhydrous CH2Cl2 (2.5 mL) and cooled by a bath of dry ice/acetone (−78 °C) under a nitrogen atmosphere. tert-Butyl 2-diazobutanoate (146 mg, 0.86 mmol) was dissolved in anhydrous CH2Cl2 (1 mL) and added to the reaction mixture over 1 hour via syringe pump. After the addition was complete, the reaction mixture was allowed to stir for an additional 5 minutes and was then allowed to warm up to room temperature. Mesitylene (61 mg, 0.51 mmol) was added to the reaction mixture and an 1H NMR spectrum was taken to estimate the yield and isomer ratio(s). The solvent was subsequently removed and the residue was chromatographed on silica gel to give 123 mg (0.39 mmol, 76%) of 5a as a colorless oil. A similar experiment starting with 50 mg (0.47 mmol) of benzaldehyde, 37 mg (0.52 mmol) of methyl vinyl ketone, and 136 mg (0.80 mmol) of tert-butyl 2-diazobutanoate gave 124 mg (0.39 mmol, 83%) of 5a. 1H NMR (400 MHz, CDCl3, δ): 7.43-7.38 (m, 2H), 7.33-7.21 (m, 3H), 5.32 (d, J = 7.2 Hz, 1H), 3.46 (app dt, J = 7.6 Hz, 3.5 Hz, 1H), 2.85 (dd, J = 13.5 Hz, 3.5 Hz, 1H), 2.07 (dd, J = 13.3 Hz, 7.4 Hz, 1H), 2.00-1.88 (m, 1H), 1.82-1.72 (m, 1H), 1.59 (s, 9H), 1.42 (s, 3H), 0.98 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3, δ): 207.2 (u), 172.6 (u), 138.1 (u), 128.4 (dn), 128.0 (dn), 126.5 (dn), 87.0 (u), 82.9 (dn), 81.0 (u), 57.0 (dn), 37.8 (u), 32.4 (u), 30.5 (dn), 28.0 (dn), 8.58 (dn); IR (CHCl3, cm−1): 2981, 2937, 1731, 1714, 1615, 1457, 1370, 1254, 1124, 1061, 908, 845, 701, 662; HRMS-ESI m/z: [M+Na], calcd for C19H26O4Na, 341.1729; found 341.1719.
Supplementary Material
Experimental and characterization details, 1H and 13C NMR spectra are provided for new compounds. A description of stereochemical assignments is provided, as are NOE experiments on 17 and on both diastereomers of compound 3c. CIF files are provided for 5b, 9a, 12 and 14. This material is available free of charge via the Internet at http://pubs.acs.org
Figure 1.
Mixing experiment
Acknowledgments
This work was supported by NIH grant GM068640. We thank Glenn Yap for X-ray crystallography.
References
- 1.(a) Gothelf KV, Jørgensen KA. Chem Rev. 1998;98:863. doi: 10.1021/cr970324e. [DOI] [PubMed] [Google Scholar]; (b) Padwa A, Hornbuckle SF. Chem Rev. 1991;91:263. [Google Scholar]; (c) Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. John Wiley; New York: 1998. [Google Scholar]; (d) Adams J, Spero DM. Tetrahedron. 1991;47:1765. [Google Scholar]; (e) Mehta G, Muthusamy S. Tetrahedron. 2002;58:9477. [Google Scholar]
- 2.(a) Padwa A, Dean DC, Osterhout MH, Precedo L, Semones MA. J Org Chem. 1994;59:5347. [Google Scholar]; (b) Doyle MP, Forbes DC, Protopova MN, Stanley SA, Vasbinder MM, Xavier KR. J Org Chem. 1997;62:7210. doi: 10.1021/jo970641l. [DOI] [PubMed] [Google Scholar]; (c) Kitagaki S, Anada M, Kataoka O, Matsuno K, Umeda C, Watanabe N, Hashimoto S. J Am Chem Soc. 1999;121:1417. [Google Scholar]; (d) Padwa A, Precedo L, Semones MA. J Org Chem. 1999;64:4079. [Google Scholar]; (e) Hamaguchi M, Matsubara H, Nagai T. J Org Chem. 2001;66:5395. doi: 10.1021/jo015618l. [DOI] [PubMed] [Google Scholar]; (f) Muthusamy M, Gunanthan C, Suresh E. Tetrahedron. 2004;60:7885. [Google Scholar]; (g) Torssell S, Somfai P. Adv Synth Catal. 2006;348:2421. [Google Scholar]; (h) Galliford CV, Scheidt KA. J Org Chem. 2007;72:1811. doi: 10.1021/jo0624086. [DOI] [PubMed] [Google Scholar]; (i) Shi J, Zhao M, Lei M, Shi M. J Org Chem. 2008;73:305. doi: 10.1021/jo701561d. [DOI] [PubMed] [Google Scholar]; (j) England DB, Eagan JM, Merey G, Anac O, Padwa A. Tetrahedron. 2008;64:988. doi: 10.1016/j.tet.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) England DB, Padwa A. J Org Chem. 2008;73:2792. doi: 10.1021/jo8001003. [DOI] [PubMed] [Google Scholar]
- 3.de March P, Huisgen R. J Am Chem Soc. 1982;104:4952.de March P, Huisgen R. J Am Chem Soc. 1982;104:4953.Alt M, Mass G. Tetrahedron. 1994;50:7435.Lu CD, Chen ZY, Liu H, Hu WH, Mi AQ, Doyle MP. J Org Chem. 2004;69:4856. doi: 10.1021/jo0497508.Skaggs AJ, Lin EY, Jamison TF. Org Lett. 2002;4:2277. doi: 10.1021/ol026149s.Muthusamy S, Gunanathan C, Nethaji M. J Org Chem. 2004;69:5631. doi: 10.1021/jo0493119.Nair V, Mathai S, Varma RL. J Org Chem. 2004;69:1413. doi: 10.1021/jo035673p. For the stereospecific synthesis of tetrahydrofuran derivatives by the Lewis acid catalyzed cycloadditions of aldehydes and donor–acceptor cyclopropanes: Pohlhaus PD, Sanders SD, Parsons AT, Li W, Johnson JS. J Am Chem Soc. 2008;130 doi: 10.1021/ja8015928.
- 4.(a) Doyle MP, Forbes DC, Protopopova MN, Stanley SA, Vasbinder MM, Xavier KR. J Org Chem. 1997;62:7210. doi: 10.1021/jo970641l. [DOI] [PubMed] [Google Scholar]; (b) Russell AE, Brekan J, Gronenberg L, Doyle MP. J Org Chem. 2004;69:5269. doi: 10.1021/jo049403y. [DOI] [PubMed] [Google Scholar]; (c) Jiang B, Zhang X, Luo Z. Org Lett. 2002;4:2453. doi: 10.1021/ol0200854. [DOI] [PubMed] [Google Scholar]; (d) Lu CD, Chen ZY, Liu H, Hu WH, Mi AQ. Org Lett. 2004;6:3071. doi: 10.1021/ol0489494. [DOI] [PubMed] [Google Scholar]; (e) Alt M, Mass G. Tetrahedron. 1994;50:7435. [Google Scholar]; (f) Wenkert E, Khatuya H. Tetrahedron Lett. 1999;40:5439. [Google Scholar]; (g) DeAngelis A, Panne P, Yap GPA, Fox JM. J Org Chem. 2008;73:1435. doi: 10.1021/jo702308f. [DOI] [PubMed] [Google Scholar]
- 5.For 3-component dioxolane formation, see: Nair V, Mathai S, Nair SM, Rath NP. Tetrahedron Lett. 2003;44:8407.Nair V, Mathai S, Mathew SC, Rath NP. Tetrahedron. 2005;61:2849.Lu CD, Chen ZY, Liu H, Hu WH, Mi AQ. Org Lett. 2004;6:3071. doi: 10.1021/ol0489494.
- 6.For the Rh-catalyzed preparation of (Z)-alkenes via β-hydride elimination, see: Taber DF, Herr RJ, Pack SK, Geremia JM. J Org Chem. 1996;61:2908. doi: 10.1021/jo952098j. and references therein.. For recent studies on suppressing β-hydride elimination through ligand selection, see ref 4h and (b) Panne P, DeAngelis A, Fox JM. Org Lett. 2008;10:2987. doi: 10.1021/ol800983y.Panne P, Fox JM. J Am Chem Soc. 2007;129:22. doi: 10.1021/ja0660195.
- 7.(a) Minami K, Saito H, Tsutsui H, Nambu H, Anada M, Hashimoto S. Adv Synth Catal. 2005;347:1483. [Google Scholar]; (b) Tsutsui H, Abe T, Nakamura S, Anada M, Hashimoto S. Chem Pharm Bull. 2005;10:1366–1368. doi: 10.1248/cpb.53.1366. [DOI] [PubMed] [Google Scholar]
- 8.For reactions of carbonyl ylides with cyclopropenes, see: Diev VV, Kostikov RR, Gleiter R, Molchanov AP. J Org Chem. 2006;71:4066. doi: 10.1021/jo0600656.
- 9.We note that functional group tolerance was initially guided by an inhibition study, in which various functionalized molecules were added in superstoichiometric amounts to the reaction between tert-butyl 2-diazohydrocinnamate, benzaldehyde and methyl vinyl ketone (see Supporting Information).
- 10.Doyle MP, Hu W, Timmons DJ. Org Lett. 2001;3:933. doi: 10.1021/ol015600x. [DOI] [PubMed] [Google Scholar]; b) Davies HML, DeMesse J. Tetrahedron Lett. 2001;42:6803. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental and characterization details, 1H and 13C NMR spectra are provided for new compounds. A description of stereochemical assignments is provided, as are NOE experiments on 17 and on both diastereomers of compound 3c. CIF files are provided for 5b, 9a, 12 and 14. This material is available free of charge via the Internet at http://pubs.acs.org