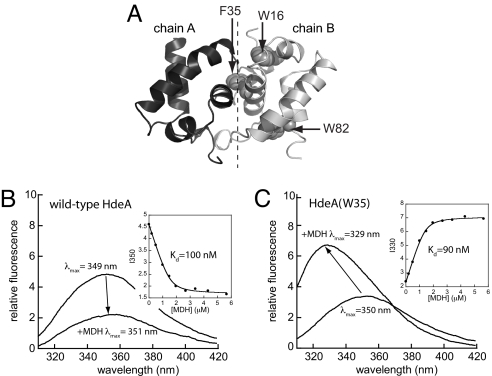
Fig. 4.
Hydrophobic amino acids at the dimer interface of inactive HdeA contribute to substrate binding in active HdeA monomers. (A) Backbone representation of the HdeA dimer (PDB ID code 1DJ8; chains A and B from ref. 12) indicating positions of the two intrinsic tryptophan residues (W16 and W82), and F35, which was used to introduce a unique tryptophan residue at the dimer interface. Emission spectra (λex = 295 nm) of 1 μM wild-type HdeA (B) or HdeA(W35) (C) were recorded in the absence or presence of 2 μM MDH. Spectra were recorded in 100 mM potassium phosphate, 150 mM NaCl (pH 2.2) at 37 °C. (Insets) Peak intensity versus MDH concentration, which were fit to determine apparent Kd values (see see SI Materials and Methods).