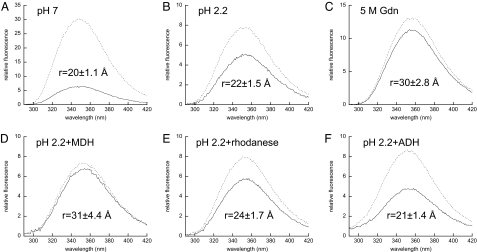
Fig. 5.
Substrate binding induces substantial conformational changes in HdeA. HdeA conformation was assessed by determining the FRET efficiency and apparent distances between the W82 donor and TNB acceptor attached via C27. Emission spectra (λex = 280 nm) of 1 μM HdeA(W82,C27) were recorded in the absence (FD, dashed lines) or presence of the TNB acceptor (FDA, solid lines) attached via C27 at pH 7.0 (A), pH 2.2 (B), or in 5 M Gdn (C). The effects of substrate binding on the conformation of active 1 μM HdeApH2.2 were assessed in the presence of 4 μM MDH (D), 4 μM rhodanese (E), or 4 μM ADH (F). Integrated emission intensities were used to calculate FRET efficiency and apparent distances (r) as outlined in SI Materials and Methods. Triplicate samples were prepared for each condition, and the resulting standard deviations were used in conjunction with errors in R0 to determine the error for each distance reported (see SI Materials and Methods).