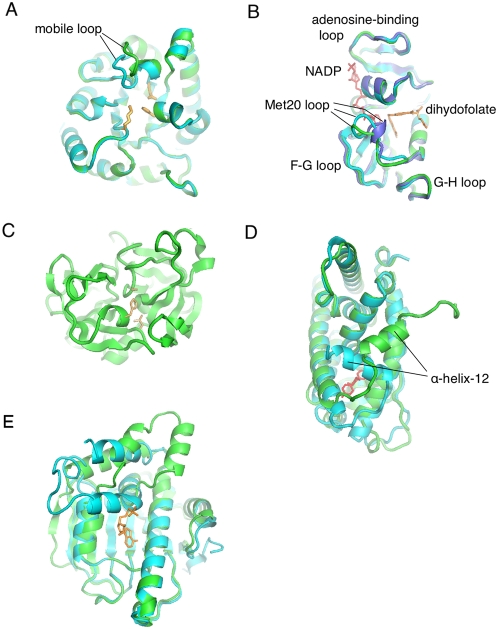
Figure 1. Structures with intermediate-scale motions.
(A) Triosephosphate Isomerase (TIM) has a mobile loop that covers the active site (orange). The mobile loop has been crystallized in both an open [8tim] (green) and closed [1TPH] (blue) conformations. (B) Dihydrofolate Reductase (DHFR) pos-sesses the Met20-loop that has been crystallized in three different states - open [1RA2] (green), closed [1RX2] (blue) and occluded [1RX7] (purple). There is experimental evidence that the Met20-loop interacts with the adenosine-binding loop, the F–G loop and the G–H loop. (C) α-Lytic protease (αLP) [1SSX] is the control as it is a kinetically-stable protease (catalytic triad in orange) that does not possess any mobile loops. (D) The Estrogen Receptor (ER) has a highly mobile Helix-12 that covers the ligand (red) binding site. ER has been crystallized in a closed [1QKU] (blue) and open [1QKT] (green) conformation. (E) The N-terminal domain of the chaperone HSP90 (HSP90) has a 23 amino acid lid [2IOR] (green) that undergoes a large conformational change to bind ADP [2IOQ] (blue).