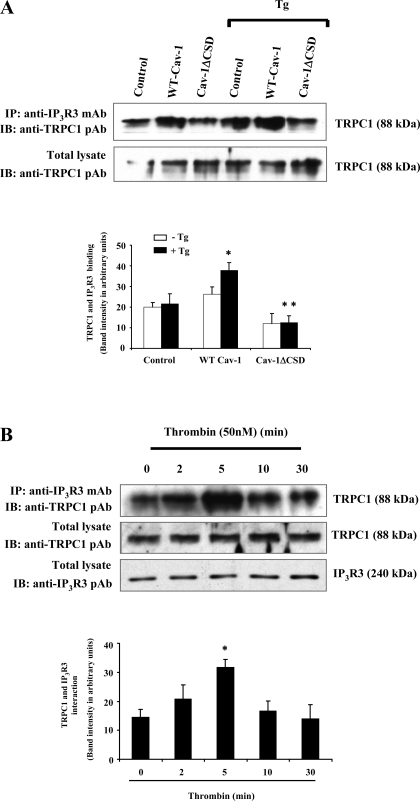
Fig. 4.
A: TG-induced Ca2+ store depletion enhances the interaction between IP3R3 and TRPC1. HEK-293 cells transfected with WT-Cav-1 or Cav-1ΔCSD were treated with or without 1 μM TG for 5 min at 37°C. Untransfected cells with or without TG treatment served as controls. After TG treatment, cells were washed and extracted proteins were immunoprecipitated using anti-IP3R3 mAb. The precipitated proteins were immunoblotted with anti-TRPC1 pAb (top). The total cell lysates were also immunoblotted with anti-TRPC1 pAb (bottom). A representative blot is shown. Immunoprecipitated protein bands were quantified by densitometry and are expressed in arbitrary units. Results are means ± SE from 4 experiments. *P < 0.05, significantly different from the control; **P < 0.001, significantly different compared with WT-Cav-1-expressed cells. B: thrombin-induced Ca2+ store depletion enhances the association of TRPC1 and IP3R3 in endothelial cells. HMEC stimulated with thrombin (50 nM) for different time intervals were lysed and immunoprecipitated with anti-IP3R3 mAb and immunoblotted with anti-TRPC1 pAb (top). Total cell lysates were immunoblotted with anti-TRPC1 pAb (middle) or anti-IP3R3 pAb (bottom). A representative blot is shown from 4 different experiments. Immunoprecipitated protein bands were quantified as in A. Results are means ± SE from 4 experiments. *P < 0.05, significantly different from the control (not stimulated with thrombin).