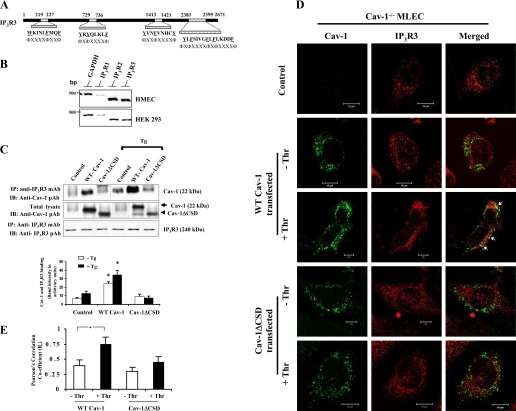
Fig. 5.
IP3R3 interacts with Cav-1 through CSD. A: CSD-binding motifs in human IP3R3. Both the NH2 and COOH termini contain CSD-binding motifs. B: expression profile of the IP3R types in HMEC and HEK-293 cells. RT-PCR was performed to determine the expression of IP3R types (see details in materials and methods). C: HEK-293 cells transfected with WT-Cav-1 or Cav-1ΔCSD expression constructs (see details in materials and methods) were treated with TG (1 μM) for 5 min at 37°C. After TG treatment, cells were lysed and the proteins were immunoprecipitated with anti-IP3R3 mAb. Cells that were not treated with TG served as controls. The precipitated proteins were immunoblotted with anti-Cav-1 pAb (top) and anti-IP3R3 pAb (bottom). Total cell lysates were immunoblotted with anti-Cav-1 pAb (middle). The TG-induced Ca2+ store depletion enhanced the binding between IP3R3 and Cav-1; however, this binding was not observed in Cav-1ΔCSD-expressing cells. The experiment was repeated 4 times, and the immunoprecipitated protein bands were quantified by densitometry. The plot shown represents means ± SE; *P < 0.05, significantly different from control. D: confocal images show the colocalization of Cav-1 with IP3R3 in control, WT-Cav-1, or Cav-1ΔCSD-expressed Cav-1−/− MLEC treated without or with 50 nM thrombin (Thr) for 5 min. Following thrombin treatment, cells were fixed and stained with anti-Cav-1 mAb (green) and anti-IP3R3 pAb (red) to visualize the localization of Cav-1 and IP3R3. This experiment was repeated 3 times, and the results are from a representative experiment. Bar, 10 μm. Arrows represent the colocalization (yellow) of Cav-1 with IP3R3. E: thrombin-induced colocalization of WT-Cav-1 or Cav-1ΔCSD with IP3R3 was quantified (see details in materials and methods). An average of five examined areas (regions of interest) were collected and plotted. *P < 0.05, significantly different from control (not stimulated with thrombin).