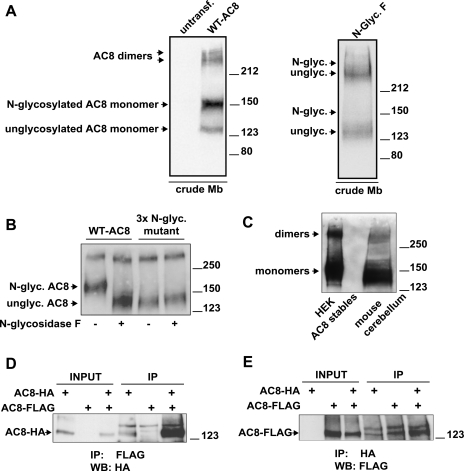
Fig. 1.
Adenyl cyclase type 8 (AC8) exists as multiple species, is N-glycosylated, and dimerizes. A: crude membrane (Mb) preparations from untransfected or transiently transfected human embryonic kidney (HEK)-293 cells were solubilized, and proteins (25 μg) were resolved by 7% acrylamide SDS-PAGE and probed for AC8 by immunoblotting. For the treatments with N-glycosidase F, crude membranes were treated as described in experimental procedures. B: crude membranes prepared from HEK-293 cells stably expressing wild-type (WT) AC8 or a triple N-glycosylation mutant of AC8 were treated or not with N-glycosidase F. Proteins (1.2 μg) were resolved by 8% acrylamide SDS-PAGE and analyzed for AC8 by immunoblotting. C: detection of endogenous AC8. Proteins (250 μg) from crude mouse cerebellar membranes were loaded on 7% acrylamide gel (SDS-PAGE), alongside 0.4 μg of proteins from HEK-293 AC8 stables as a positive control, and analyzed for expression of AC8 by immunoblotting. D and E: coimmunoprecipitation of COOH-terminally hemagglutinin (HA)- or FLAG-tagged AC8 molecules (AC8-HA and AC8-FLAG), transiently expressed alone or in combination. Each immunoprecipitation was performed on 0.5–1 mg of precleared crude membrane proteins. The immunoprecipitated proteins were eluted from the beads and analyzed by 7% acrylamide SDS-PAGE and immunoblotting with the indicated antibodies (at 1:5,000 dilution). Inputs were 5% of the precleared crude membrane proteins used for immunoprecipitation. IP, immunoprecipitation; WB, immunoblotting.