Figure 6.
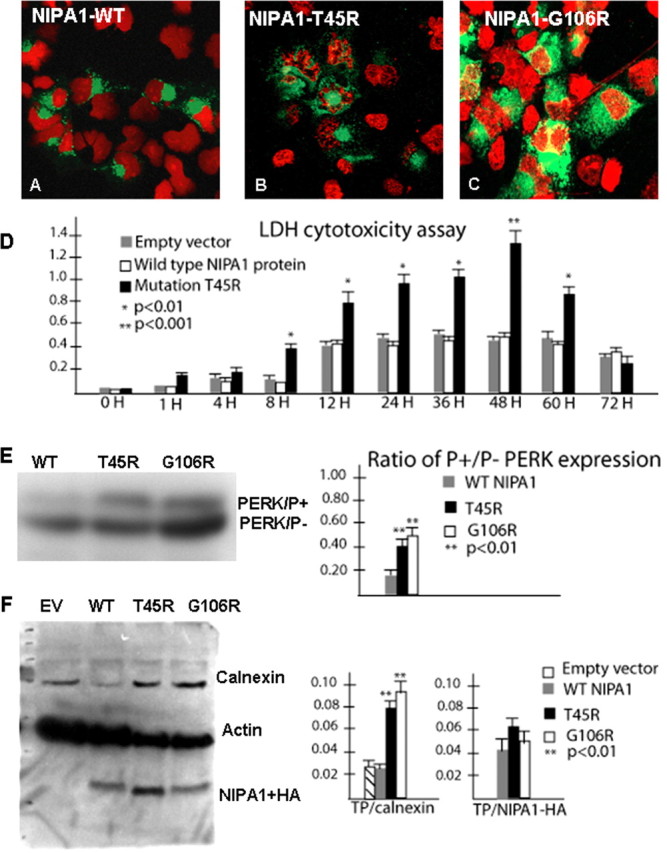
Mutant NIPA1 induces ER stress and program cell death. Transfection with wild-type NIPA1::EGFP showed normal nuclear structure (in red) after staining with 4′,6-diamidino-2-phenylindole dihydrochloride (A, EGFP signal indicated the transfection of the cell). Transfection with mutant T45R (B) and G106R (C) induced apoptotic cell death, indicated by a fragmented nuclear morphology caused by DNA condensation. D, The LDH-cytotoxicity assay performed on transfected HEK293 cells with WT NIPA1 and mutant (T45R) NIPA1 demonstrates a significant increase of released LDH from cells expressing mutant NIPA1 starting 8 h after the transfection and culminating between 48 and 60 h; the LDH levels dropped significantly 12 h later (gray bar, empty vector; white bar, WT NIPA1; and black bar, T45R mutation). More than 95% of transfected cells underwent apoptotic cells death 72 h after transfection. The survival of cells expressing the wild-type form of protein did not differ from cells transfected by an empty vector. The vertical axis shows values of the absorbance of the samples at 495 nm. Expression of the G106R mutation induced the comparable degree of programmed cell death (data not shown). We also assayed the markers of ER stress leading to the UPR and found a significantly increased ratio of phosphorylated pancreatic ER kinase (PERK) in the presence of either studied mutation (E; gray bar, WT NIPA1; black bar, T45R; and white bar, G106R mutation) and increased expression of calnexin [F, empty vector (EV)] compared with actin (TP). In both instances, the G106R mutation showed slightly higher activity of ER stress markers but it did not reach statistical significance.
