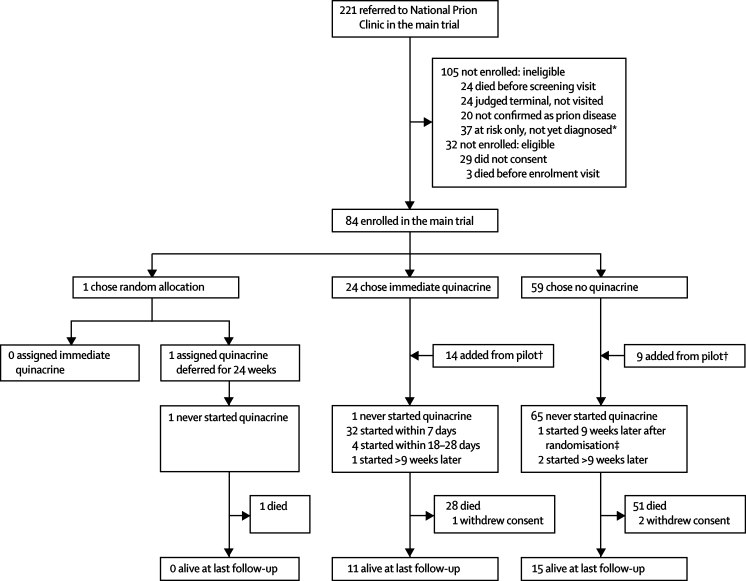
Figure 1.
Trial profile
*Relative with symptomatic inherited prion disease or recipient of blood transfusion. †From September, 2001–June, 2002, six patients received open-label quinacrine in an initial pilot study, and from August, 2002, to March, 2004, 17 more were offered quinacrine or no quinacrine in an extended pilot study (total 23 patients). ‡One originally chose not to take quinacrine but later agreed to randomisation 9 weeks after enrolment and was allocated immediate quinacrine.