Abstract
Silymarin, an extract from seeds of Silybum marianum, is used by 8-33% of patients to self-treat chronic viral hepatitis C in the United States. Studies in humans and rodents suggest that biliary excretion of glucuronide and sulfate conjugates is the major route for silymarin’s elimination. To determine the role of multidrug resistance associated-protein 2, Mrp2 (Abcc2), in the biliary excretion of silymarin, the hepatobiliary disposition of the six major silymarin flavonolignans was studied using isolated perfused livers (IPRLs) from wild-type (WT) and Mrp2-deficient (TR-) Wistar rats. For all flavonolignans, approximately 96% of the dose was cleared from perfusate within 30 min in both WT and TR- livers, and < 5% of parent was recovered in bile or perfusate by the end of the perfusion. In WT livers, the percentage of dose excreted as conjugates into bile varied for each flavonolignan (silychristin, 51.6% ± 9.3; silydianin, 101.5% ± 28.3; silybin A, 21.0% ± 8.3; silybin B, 31.7% ± 13.2; isosilybin A, 50.5% ± 23.6; isosilybin B, 42.8% ± 19.3). Among the flavonolignans, only silydianin was primarily glucuronidated and almost completely recovered in bile. In TR- livers, biliary excretion of flavonolignan conjugates was reduced 80-92%, with 30-83% of each flavonolignan conjugate recovered in perfusate compared to only 5-30% at 90 min. Biliary excretion of glucuronide and sulfate conjugates of all flavonolignans were reduced by 94-98% and 73-84% respectively, in TR- IPRLs. These data indicate a primary role for Mrp2 in the biliary elimination of silymarin flavonolignan conjugates.
Silymarin, a herbal extract commonly known as milk thistle, is used approximately by 8-33% of patients in the United States to self-treat chronic hepatitis C virus (HCV) (Seeff et al., 2008). Silymarin is a complex mixture of six major flavonolignans (silybin A and B; isosilybin A and B; silychristin; silydianin), the most common class of flavonoids in milk thistle extract, as well as other polyphenolic compounds (Kim et al., 2003). Silybin A and silybin B are the most abundant flavonolignans in milk thistle extracts and are used to standardize commercial preparations. Silymarin is hepatoprotective in laboratory animals to the toxic effects of carbon tetrachloride, ethanol and acetaminophen (Crocenzi and Roma, 2006). Recently, two independent laboratories have demonstrated the antiviral effects of silymarin in HCV replicon assays (Polyak et al., 2007; Bonifaz et al., 2007). However, the beneficial effects of silymarin in liver disease have not been established due to inconsistencies in results obtained from several randomized clinical trials that may reflect various trial designs and the use of different formulations (Mayer et al., 2005). In addition, recent data suggest that the pharmacokinetics of silymarin is influenced by the type and stage of liver disease (Schrieber et al., 2008a). While the precise pathways of metabolism and excretion have not been identified, limited data from human and animal studies suggest that flavonolignans primarily undergo rapid and extensive phase II metabolism and biliary excretion (Schandalik et al., 1992; Morazzoni et al., 1992; Morazzoni et al., 1993).
In rats and humans, silymarin is characterized by low bioavailability, rapid conjugation and biliary excretion of silymarin and flavonolignan conjugates. In rats, only 2% of the orally administered dose of silymarin was recovered in the bile unchanged while the remaining was recovered as sulfate and glucuronide conjugates (Morazzoni et al., 1993). However, the transport proteins primarily responsible for biliary excretion of silymarin and its metabolites have not been identified.
Silymarin is a potent inhibitor of breast cancer resistance protein (Bcrp) in MCF-7 cells (Zhang et al., 2004). Additionally, it has been shown that silymarin can inhibit P-glycoprotein-mediated efflux of digoxin in human intestinal Caco-2 cells (Zhang and Morris, 2003). Multidrug resistance associated-protein, Mrp2, which is expressed in the liver, gut, kidney, and brain mediates the biliary elimination of many flavonol conjugates (Hoffmann and Kroemer, 2004; Cermak and Wolffram, 2006). Mrp2 (Abcc2) is a member of the ATP-binding cassette (ABC) superfamily of membrane transporters which is responsible for the biliary excretion of organic anions, glucuronide, glutathione and sulfate conjugates of endogenous compounds as well as xenobiotics (Zamek-Gliszczynski et al., 2005; Zamek-Gliszczynski et al., 2006a; König et al., 1999). The specific role of each of these canalicular transport proteins in the excretion of silymarin and its conjugates has not been established.
The isolated perfused rat liver (IPRL) is a valuable and commonly employed ex vivo model for studying the metabolism and transport mechanisms involved in the hepatobiliary disposition of drugs. This model permits exposure of the liver to different test substances, allows repeated sampling to determine disposition of parent compound and metabolites in perfusate and bile, and is independent of the influence of other organ systems (Gores et al., 1986). Compared with isolated hepatocytes, hepatic architecture, cell polarity and bile flow are preserved in the isolated perfused liver (Jansen et al., 1985), making this a useful model to study metabolism and biliary excretion of various substances. The IPRL technique has been used successfully in various genetic animal models to investigate the precise mechanisms of hepatobiliary disposition of drugs. For example, a number of studies have employed IPRL to examine the role of Mrp2 utilizing TR- rats which are mutant rats that lack functional Mrp2 at the canalicular membrane (Jansen et al., 1985; McDonagh et al., 2001), similar to patients with Dubin-Johnson syndrome (Paulusma et al., 1997). Such studies have demonstrated marked reduction in biliary excretion of glucuronide and sulfate conjugates of drugs and other compounds in TR- rats, implying that Mrp2 is responsible for the biliary excretion of organic anions.
The purpose of this study was to determine the role of Mrp2 in the biliary excretion of the six major silymarin flavonolignans and their conjugates by using IPRLs from Mrp2-competent (wild-type) and Mrp2-deficient (TR-) Wistar rats. Pharmacokinetic parameters were determined for both the perfusate and bile compartments.
Materials and Methods
Chemicals
Silymarin (SIL), dimethyl sulfoxide (DMSO), β-glucuronidase (EC 3.3.1.3.1; type B-10 from bovine liver), sulfatase (EC3.1.6.1; type H-1 from Helix pomatia), D-saccharic acid 1,4-lactone (D-SL), naringenin, and taurocholate were purchased from Sigma-Aldrich (St. Louis, MO). Reference standards for silychristin (SC) were obtained from ChromaDex (Santa Ana, CA), and silydianin (SD) was purchased from U.S. Pharmacopoeia (USP; Rockville, MD). The composition of silybin (Silibinin, Sigma-Aldrich, St. Louis, MO) was confirmed to be a mixture of silybin A (SA) and silybin B (SB) by LC-ESI-MS and the specific contents of SA and SB were analyzed to be 48% and 52%, respectively. Isosilybin A (ISA) and isosilybin B (ISB) reference standards were obtained as a generous gift from Ulrich Mengs (Madaus GmbH, Germany). The purity of all six silymarin flavonolignan standards was between 97- 99%. All other chemicals and reagents were of analytical grade and available from commercial sources.
Animals
Male Wistar rats (200-250 g) from Charles River Laboratories, Inc. (Raleigh, NC), and male TR- rats bred in our facility (275-300 g; breeding stock obtained from Dr. Mary Vore, University of Kentucky, Lexington, KY) were used as liver donors for perfused liver studies. Retired male Wistar breeder rats (400-500 g; Charles River Laboratories, Inc.) were used as blood donors. Rats were housed in an alternating 12-h light/dark cycle with rat chow and water provided ad libitum. All animals were allowed to acclimate for at least one week before experimentation. Rats were anesthetized with ketamine/xylazine (60:12 mg/kg i.p). The Institutional Animal Care and Use Committee at the University of North Carolina approved all animal procedures.
Liver Perfusion Studies
Recirculating IPRL studies were conducted with wild-type (WT) and TR- rat livers as described previously (Brouwer and Thurman, 1996). Briefly, after portal vein and bile duct cannulation, livers were perfused in situ with oxygenated buffer (pH 7.4). Livers were removed from the body cavity and placed in a humidified perfusion chamber heated to maintain liver temperature at 37°C. Perfusion was continued with oxygenated Krebs-Henseleit bicarbonate buffer containing 20% (v/v) heparinized male rat blood at a flow rate of 20 ml/min (80 ml, total volume). Livers and perfusate were allowed to acclimate 10 min before addition of 26 μg/ml silymarin. Liver viability was assessed by monitoring portal pressure (<15 cm of H2O), observing gross morphology, and measuring initial bile flow in WT and TR- IPRLs (>0.8 and >0.3 μl/min/g liver, respectively). Taurocholate (0.5 μmol/min, in saline) was infused into the perfusate reservoir to maintain bile flow. Bile was collected continuously at 10-min intervals, and the volume was determined gravimetrically (specific gravity 1.0). Perfusate (0.5 ml) was sampled every 10 min and immediately centrifuged to obtain supernatant for analysis. After perfusion, livers were blotted dry and weighed. All samples collected from this experiment were stored at -80°C until further analysis.
Analysis of Silymarin Flavonolignans in Perfusate and Bile
Identification and quantification of silymarin flavonolignans in the perfusate and bile was carried out by LC-ESI-MS using a previously established method with slight modifications (Wen et al., 2008). Briefly, separation of silymarin flavonolignans was performed using an Agilent HP 1100 LC system with a C18 SecurityGuard cartridge (4 × 2.0 mm i.d.; Phenomenex, Torrance, CA) and a Luna C18 (2) analytical column (50 × 2.0 mm i.d., 3 μm; Phenomenex, Torrance, CA). HPLC conditions were as follows: mobile phase, methanol: 1% glacial acetic acid (pH 2.8) (43:57, v/v) with isocratic elution; flow rate, 0.3 ml/min; injection volume, 25 μl; run time, 13 min. Typical retention times of silymarin flavonolignans SC, SD, SA, SB, ISA, ISB, and naringenin (internal standard), under the experimental conditions were 1.8, 2.1, 4.4, , 5.5, 7.4, 8.2, and 5.0 min, respectively. MS analysis and detection were conducted by a HP 1100 LC-MSD system (Agilent Technologies, Palo Alto, CA) with an electrospray interface in the negative ESI ionization mode. MS parameters used for quantitative analysis were: capillary voltage, -3500 V; fragmentor voltage, 150V; drying gas temperature, 350 °C; nebulizer gas pressure, 35psig; drying gas flow, 8 l/ml; scan mode, selective ion monitoring (SIM) with [M-H]- for silymarin flavonolignans (m/z 481), silymarin sulfates (m/z 561), silymarin glucuronides (m/z 657), and naringenin (m/z 271), respectively. Split calibration curves for the free (unconjugated) and total (unconjugated plus conjugated) flavonolignans in the concentration range of 10-500 ng/ml and 500-10,000 ng/ml were generated using reference standards of SC, SD, SA, SB, ISA and ISB. Mixed standard solutions containing SC, SD, SA, SB, ISA, and ISB were spiked into blank perfusates and bile treated as described in the perfusate and bile sample preparation (see below). Concentrations of silymarin flavonolignans in the samples were estimated with 1/x2 weighted least-squares regression equations derived from the peak area ratios of individual silymarin flavonolignans to that of the internal standard. The limit of detection was 5 ng/ml and limit of quantification for the silymarin flavonolignans was 10 ng/ml. Intra- and inter-day precisions were 1.7 - 11%, and 4.5 - 14%, respectively.
Perfusate and bile samples were initially diluted 1:5 and 1:50, respectively. Aliquots of perfusate and bile samples as well as blank perfusate and bile spiked with standards of SC, SD, SA, SB, ISA and ISB, were protein-precipitated by the addition of 300 μl of ice-cold acetonitrile and 1% glacial acetic acid containing internal standard, naringenin (200 ng). After the removal of protein by centrifugation at 15,000g for 15 min at 4°C, the supernatants were transferred and evaporated with a gentle stream of nitrogen at 45°C in a water bath. The residue was reconstituted in 100 μl of the HPLC mobile phase and centrifuged at 10,000g for 10 min at 4°C; 25 μl of the reconstituted supernatants were introduced for LC-ESI-MS analysis.
Enzyme Hydrolysis of Sulfate and Glucuronide Conjugates of Silymarin
Concentrations of sulfated, glucuronidated, and total (unconjugated and conjugated) silymarin flavonolignans in perfusate and bile were measured after hydrolysis with sulfatase, β-glucuronidase and a mixture of sulfatase and β-glucuronidase, respectively (Wen et al., 2008). Unconjugated silymarin flavonolignan concentrations in perfusate and bile were directly determined without enzyme hydrolysis. Conjugated silymarin flavonolignan concentrations were determined as the difference between total (determined with enzyme hydrolysis) and unconjugated (without enzyme hydrolysis) and expressed as parent equivalents of silymarin flavonolignans. Briefly, aliquots of 100 μl diluted perfusate and bile were treated with sulfatase (80 U/ml in the final incubation) containing D-SL (10mM), β-glucuronidase containing 0.1 M phosphate buffer (8000 U/ml in the final incubation), and a mixture of sulfatase (80 U/ml) and β-glucuronidase (8000 U/ml), respectively. The individual mixtures with different hydrolytic enzymes were buffered by 0.25 M sodium acetate (pH 5.0) and incubated (final volume 120 μl) at 37°C with gentle shaking for 6 h. The reactions were terminated by the addition of 300 μl of ice-cold acetonitrile containing 1% glacial acetic acid and internal standard naringenin (200 ng). After the removal of protein by centrifugation at 15,000g for 15 min at 4°C, the supernatants were evaporated and treated as described in the analysis of silymarin flavonolignans in perfusate and bile; the final reconstituted samples were analyzed by LC-ESI-MS.
Pharmacokinetics and Statistical Analyses
The six major flavonolignans comprised 2.08 mg or 53.88% of the 3.86 mg milk thistle extract added to the 80 ml of perfusate. Initial quantities of the six flavonolignans added to the perfusate were as follows: 470 μg of silychristin, 188 μg of silydianin, 443 μg of silybin A, 726 μg of silybin B, 172 μg of isosilybin A and 81 μg of isosilybin B (Wen et al., 2008). Thus, the initial total perfusate concentration of flavonolignans was 26 μg/ml. All data are expressed as mean ± S.E.M, from three individual experiments. Pharmacokinetic parameters for individual silymarin flavonolignans (unconjugated and conjugates, respectively) were calculated using WinNonlin 4.1 (Pharsight Corporation, Mountain View, CA). The individual unconjugated silymarin flavonolignan concentrations over 90 min in the perfusate and extrapolated to infinity were used to calculate the clearance (CL) from the perfusate, and the estimated initial perfusate concentration (C0), using a non-compartmental i.v. model. The area under the curve during the 90-min perfusion (AUC0→90 min) was calculated according to the log-linear trapezoidal rule. Biliary clearance (Clb) for each silymarin flavonolignan during the 90-min collection interval was calculated as the cumulative amount of unconjugated flavonolignan excreted in the bile (Ae) divided by the perfusate AUC0→90 min of the unconjugated flavonolignans. Apparent hepatobiliary clearance of conjugated silymarin flavonolignans was defined as the cumulative amounts of conjugated flavonolignans excreted into the bile (Ae) divided by the plasma AUC0→90 min for each of the flavonolignans. The following assumptions are included in the definition of hepatobiliary clearance: 1) conjugates excreted into the perfusate return to the liver for biliary excretion; and 2) there is no competition between flavonolignans and flavonolignan conjugates for biliary excretion. Thus, the apparent hepatobiliary clearance encompasses the formation clearance to conjugates that are ultimately excreted in bile as well as biliary excretion of conjugates. Statistical differences between WT and TR- IPRL data were assessed using ANOVA followed by Student’s t-test at a significance level of p<0.05.
Results
Pharmacokinetics of Silymarin Flavonolignans in Isolated Perfused Rat Liver
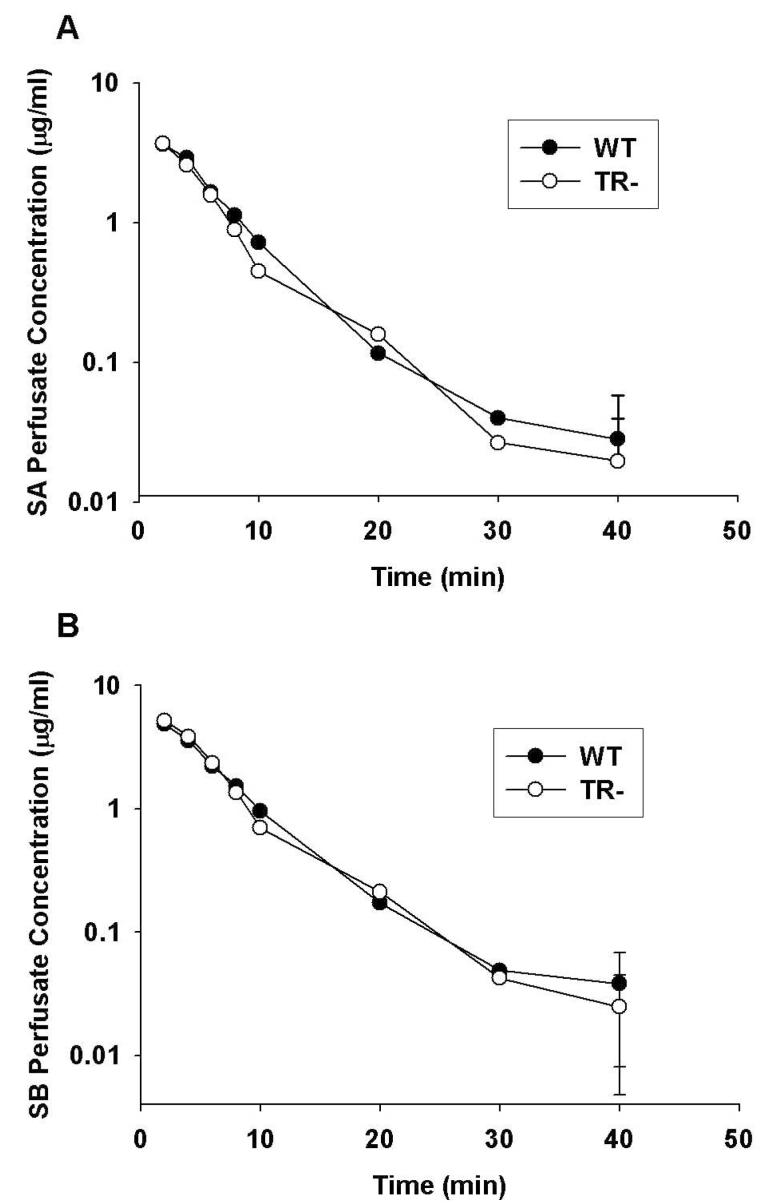
Pharmacokinetic parameters describing the disposition of the six major silymarin flavonolignans in the WT and TR- IPRLs during the 90-min perfusion are presented in Table 1. In addition, the complete concentration-time profiles from which pharmacokinetic data were obtained for silybin A and silybin B are depicted in Figure 1, since they comprise approximately 60% of the total flavonolignans in silymarin extracts and are the most well studied flavonolignans in vivo. As shown in Figure 1, similar perfusate concentration-time profiles were obtained from WT and TR- IPRLs; approximately 97% of silybin A and silybin B were cleared from the perfusate by 30 min. Similar concentration-time profiles also were observed for silychristin, silydianin, isosilybin A and isosilybin B in both WT and TR- IPRLs (data not shown). When the extrapolated initial perfusate concentrations (C0) for each of the six flavonolignans were summed, the total concentration of silymarin flavonolignans in the perfusate amounted to 21.9 μg/ml for WT and 21.3 μg/ml for TR- IPRLs, which were comparable to the theoretical perfusate silymarin concentration of 26 μg/ml at the start of each perfusion. Consistent with the concentration-time profiles, no significant differences in the clearance of each silymarin flavonolignan from the perfusate were observed between WT and TR- IPRLs. These data suggest that the exposure of WT and TR- IPRLs to each flavonolignan was similar, allowing for mass-balance comparisons.
Table1.
Pharmacokinetic Parameters of Unconjugated Silymarin Flavonolignans in Perfusate of WT and TR- Rats.
| Flavonolignan | C0 (μg/mL) | AUC(min*μg/mL) | Cl (mL/min) | Clb (mL/min) | ||||
|---|---|---|---|---|---|---|---|---|
| WT | TR- | WT | TR- | WT | TR- | WT | TR- | |
| Silychristin | 4.59 ± 0.34 | 4.00± 0.36 | 55.66 ± 11.76 | 47.03 ± 2.15 | 8.41 ± 1.81 | 9.20 ± 0.97 | 0.15 ± 0.04 | 0.18 ± 0.05 |
| Silydianin | 2.81 ± 0.46 | 2.07 ± 0.57 | 23.47 ± 8.53 | 13.99 ± 3.69 | 9.51± 2.85 | 15.27 ± 4.04 | 0.23 ± 0.11 | 0.20 ± 0.03 |
| Silybin A | 4.95 ± 0.77 | 5.21 ± 1.20 | 31.93 ± 1.70 | 28.29 ± 3.64 | 13.35 ± 0.71 | 15.42 ± 1.58 | 0.06 ± 0.03 | 0.01 ± 0.00 |
| Silybin B | 6.65 ± 0.71 | 7.01 ± 1.50 | 40.95 ± 2.95 | 40.57 ± 4.82 | 17.06 ± 0.88 | 18.04 ± 2.02 | 0.06 ± 0.03 | 0.01 ± 0.00 |
| Isosilybin A | 1.99 ± 0.22 | 2.12± 0.60 | 10.73 ± 0.92 | 9.69 ± 1.81 | 14.76 ± 1.77 | 18.13 ± 3.02 | 0.04 ± 0.01 | 0.02 ± 0.01 |
| Isosilybin B | 0.87 ± 0.14 | 0.87 ± 0.23 | 6.96 ± 1.18 | 4.47 ± 1.01 | 9.12 ± 2.10 | 17.70 ± 3.40 | 0.06 ± 0.00 | 0.05 ± 0.02 |
Pharmacokinetic parameters of unconjugated silymarin flavonolignans after addition of silymarin (26 μg/ml) to the perfusate. Cl, clearance of unconjugated flavonolignans from perfusate; Clb, biliary clearance of the unconjugated flavonolignans. Data are expressed as mean ± S.E.M (n=3).
Figure 1.
Perfusate concentration versus time profiles of unconjugated silybin A (A) and silybin B (B) in isolated perfused livers from wild-type (•) and TR- (○) rats. Silymarin (26 μg/ml) was added to the recirculating perfusate reservoir and livers from male Wistar rats were perfused for 90 min. Concentrations of silybin A and silybin B were determined by LC-MS analysis as described in Materials and Methods. Data are expressed as mean of n=3 livers in each group; mean ± S.E.M at the 90-min timepoint.
Concentration-time Profiles of Flavonolignan Conjugates in Perfusate
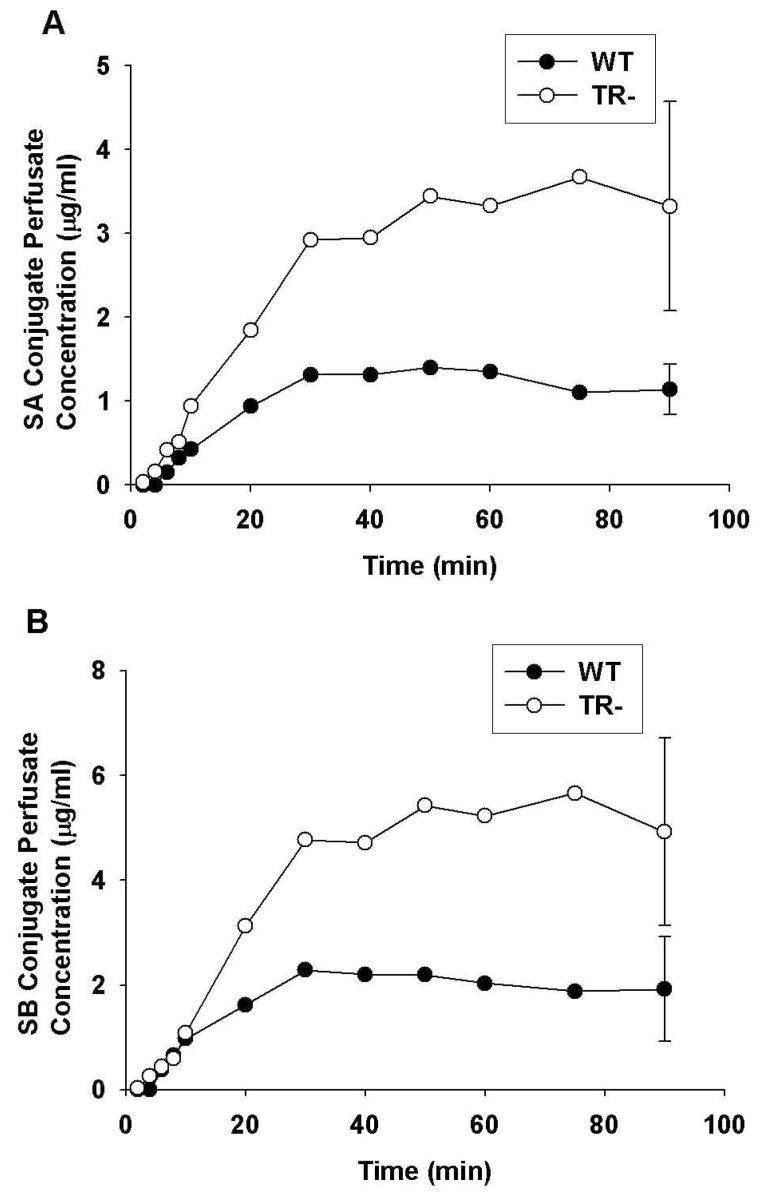
Concentration-time profiles for silybin A and silybin B conjugates in the perfusate are shown in Figure 2A and 2B, respectively. After an initial lag time of about 6 min, concentrations of flavonolignan conjugates increased rapidly in perfusate and reached a plateau after ∼ 50 min. The concentrations of silybin A and silybin B as conjugates in the perfusate of WT IPRLs at 90 min was 1.2 μg/ml and 1.9 μg/ml, respectively, or approximately 21-22% of the initial dose of the parent flavonolignans. Similar concentration-time profiles were observed for the other flavonolignans (data not shown). Perfusate conjugate concentrations of isosilybin A (0.6 μg/ml), isosilybin B (0.1 μg/ml), silychristin (0.3 μg/ml) and silydianin (0.1 μg/ml) at 90 min accounted for approximately 28%, 10%, 5%, and 4% of the dose for each flavonolignan, respectively.
Figure 2.
Perfusate concentration versus time profiles of silybin A (A) and silybin B (B) conjugates (glucuronide + sulfate) in isolated perfused livers from wild-type (•) and TR- (○) rats. Silymarin (26 μg/ml) was added to the recirculating perfusate reservoir and perfusate samples were treated with or without a mixture of β-glucuronidase and sulfatase to obtain the total and unconjugated flavonolignan concentrations, respectively. Perfusate conjugate concentrations of silybin A and silybin B were determined as the difference between the total and unconjugated concentrations for each flavonolignan. Data are expressed in equivalents of the unconjugated flavonolignans as mean of n=3 livers in each group; mean ± S.E.M at the 90-min timepoint
In contrast to WT IPRLs, approximately 78-80% of the dose for silybin A and silybin B was excreted into perfusate as conjugates at 90 min in TR- IPRLs. Similar conjugate concentration-time profiles were observed for the other flavonolignans (data not shown).
Biliary Excretion of Silymarin Flavonolignans and Flavonolignan Conjugates
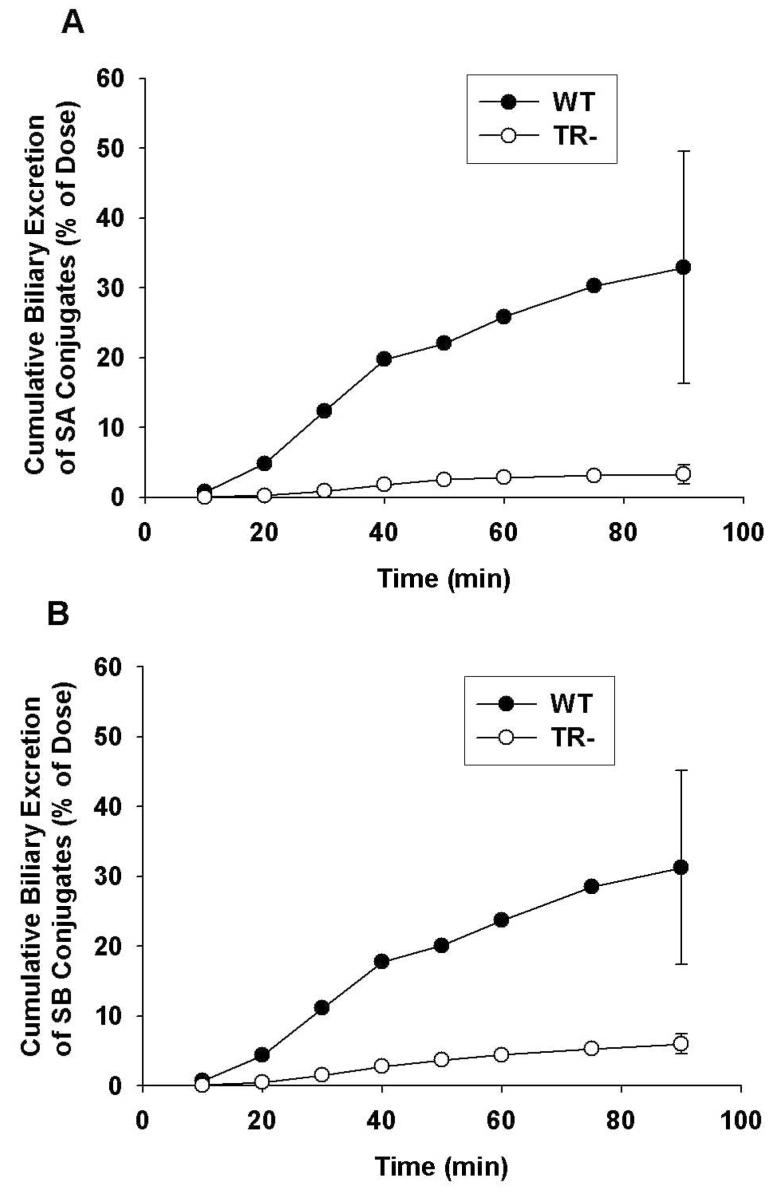
Representative cumulative biliary excretion profiles of silybin A and silybin B conjugates by WT and TR- IPRLs are shown in Figures 3A and 3B, respectively. Similar biliary excretion profiles also were obtained for silychristin, silydianin, isosilybin A and isosilybin B (data not shown). For WT IPRLs, the cumulative biliary excretion profiles for silybin A and silybin B revealed a time lag between 10 to 20 min after which the amount of flavonolignan conjugates excreted into bile rose rapidly until 40 min. This lag period coincided with the 20-min period where most of the silybins are extracted from the perfusate and into the liver (see Fig.1.), and flavonolignan conjugate concentrations plateau in the perfusate (see Fig.2.). Also depicted in Figure 3 are the cumulative biliary excretion profiles for silybin A and silybin B conjugates during perfusions of TR- livers. Perfusate concentrations of silybin conjugates rose more rapidly during the first 20 min of the perfusion compared to that observed with WT IPRLs (see Fig.2.). Only, 2.5% and 4% of the doses for silybin A and silybin B, respectively, were excreted into bile as conjugates in TR- IPRLs at 90 min compared to 21% and 32%, respectively, with WT IPRLs. In addition, the biliary excretion profiles for silychristin, silydianin, isosilybin A and isosilybin B also were significantly reduced in TR- livers (data not shown).
Figure 3.
Cumulative biliary excretion of silybin A (A) and silybin B (B) conjugates (glucuronide + sulfate) in isolated perfused livers from wild-type (•) and TR- (○) rats. Data are expressed in equivalents of the unconjugated flavonolignans as mean of n=3 livers in each group; mean ± S.E.M at the 90-min timepoint.
For WT livers, 7.2 μg of silychristin, 2.6 μg of silydianin, 2.9 μg of silybin A, 3.5 μg of silybin B, 0.5 μg of isosilybin A, and 0.3 μg of isosilybin B were excreted unchanged into the bile at the end of the 90-min perfusion. Similar amounts of each flavonolignan also were excreted into the bile by TR- IPRLs, and no differences in the biliary clearances of the unconjugated flavonolignans between livers from WT and TR- rats were evident (Table 1). These data suggest that biliary excretion of the parent flavonolignans may not be mediated by Mrp2.
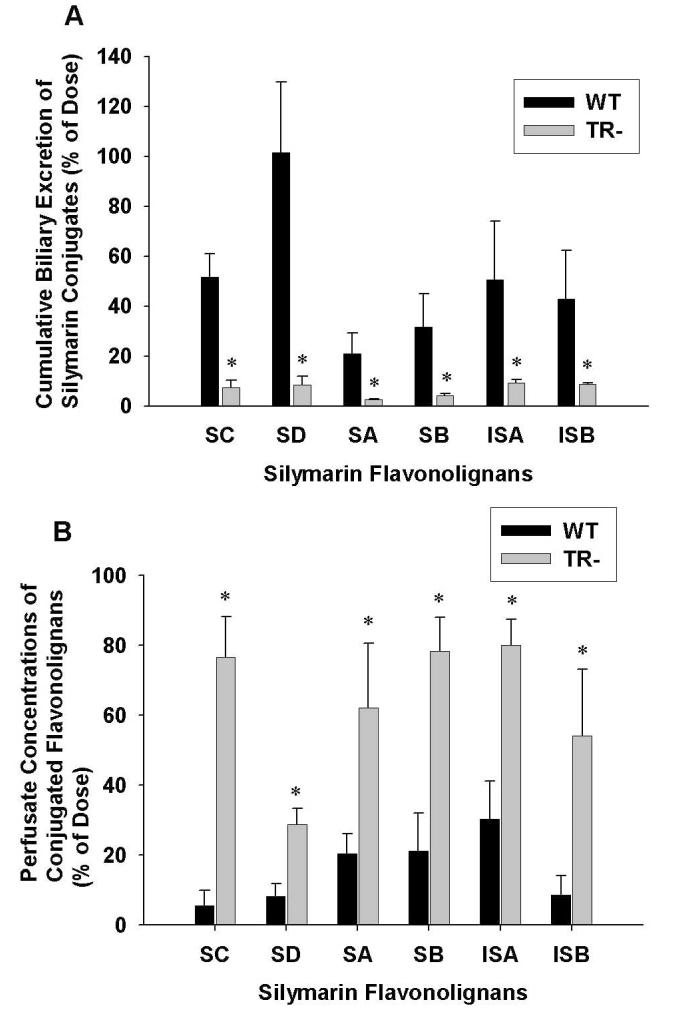
Figure 4A depicts the cumulative percentage of the dose excreted in bile through 90 min as conjugates in WT and TR- IPRLs for each of the six silymarin flavonolignans. For WT livers, 242.7 μg of silychristin, 190.8 μg of silydianin, 93.1 μg of silybin A, 230.3 μg of silybin B, 86.9 μg of isosilybin A, and 34.7 μg of isosilybin B were excreted in the bile as conjugates at the end of the 90-min perfusion. Compared to WT IPRLs, the biliary excretion of flavonolignan conjugates was reduced by 80% to 92% in TR- IPRLs (silychristin, 86%; silydianin, 92%; silybin A, 88%; silybin B, 88%; isosilybin A, 82%; and isosilybin B, 80%). These results suggest that the biliary excretion of flavonolignan conjugates is primarily dependent on Mrp2. Figure 4B summarizes the percentage of the dose recovered as conjugates (glucuronide and sulfate) in the perfusate after 90-min in both WT and TR- IPRLs for each of the six silymarin flavonolignans. Among the flavonolignans, between 30% to 83% of the administered dose was excreted into the perfusate in TR- IPRLs compared to only 5% to 30% of the dose in WT IPRLs. Silychristin conjugates exhibited the greatest increase in perfusate exposure in TR- IPRLs. However, the total amount of flavonolignan conjugates (glucuronides and sulfates) excreted in both bile and perfusate after 90-min was not different between TR- IPRLs (1552.8 μg ± 321.6) and WT IPRLs (1227.1 μg ± 265.3).
Figure 4.
Cumulative excretion of conjugated (glucuronide + sulfate) silymarin flavonolignans in bile (A) and perfusate (B) at 90 min in isolated perfused livers from wild-type and TR- rats. Data are expressed in equivalents of the unconjugated silymarin flavonolignans as mean ± S.E.M of n=3 livers in each group; *, p<0.05, WT vs TR-.
The total recovery of the 2.08 mg perfusion dose of silymarin flavonolignans ranged between 50 to 74% for WT IPRLs (mean 1.26 ± 0.26 mg), and between 64 to 91% (mean 1.60 ± 0.28 mg) for TR- IPRLs. Another 7 to 9% of the silymarin flavonolignan dose was recovered in three perfused livers that were examined (data not shown). These data suggest that the major effect of Mrp2 deficiency on the excretion of flavonolignan conjugates in rat IPRLs is a shift in excretion from bile to the perfusate.
Cumulative Biliary Excretion of Silymarin Glucuronide and Sulfate Conjugates
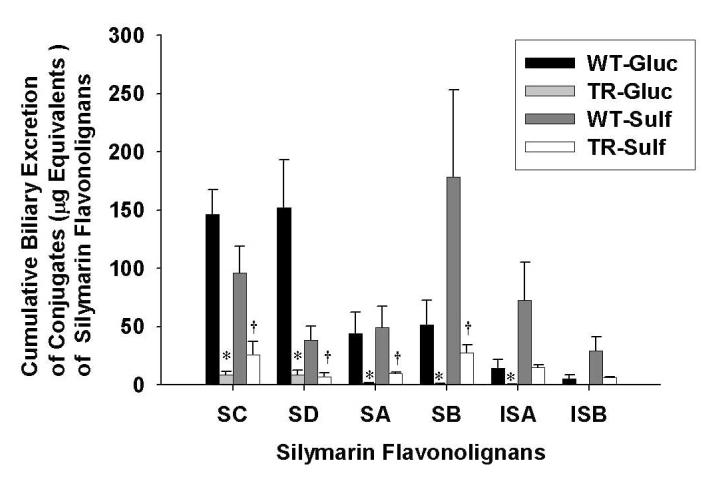
To determine whether the extent of biliary excretion of each silymarin flavonolignan was governed by the type of conjugation reaction, the cumulative biliary excretion of flavonolignan glucuronide and sulfate conjugates was determined. As shown in Figure 5 in the WT IPRLs, the primary route of conjugation for silydianin was by glucuronidation (80.5%) while sulfation was the major pathway for silybin B (78%), isosilybin A (84%) and isosilybin B (85%). Approximately equal amounts of glucuronide and sulfate conjugates were recovered in bile for silybin A and silychristin. These data suggest that each silymarin flavonolignan has a preferred route of phase II metabolism. Also depicted in Figure 5 is the cumulative biliary excretion of glucuronide and sulfate conjugates for each flavonolignan by TR- IPRLs, which were significantly reduced irrespective of the type of conjugation reaction. Compared to WT IPRLs, the amount of glucuronide and sulfate conjugates for each of the flavonolignans excreted into bile was reduced between 94-98% and 73-84%, respectively, in TR- IPRLs.
Figure 5.
Cumulative biliary excretion of the glucuronide and sulfate conjugates of silymarin flavonolignans in isolated perfused livers from wild-type and TR- rats. Data are expressed in equivalents of unconjugated silymarin flavonolignans as mean ± S.E.M of n=3 livers in each group; *, p<0.05, WT vs TR- for glucuronide conjugates; †, p<0.05, WT vs TR- for sulfate conjugates.
The apparent hepatobiliary clearances for the six flavonolignans are summarized in Table 2. Consistent with the data presented in Figure 5, hepatobiliary clearances for both glucuronide and sulfate conjugates of each silymarin flavonolignan were markedly reduced by ∼ 89-99 % and 75-90%, respectively, in TR- compared to wild-type IPRLs. The apparent hepatobiliary clearances for silydianin more closely reflect its formation clearance since this flavonolignan is almost completely excreted in the bile. Collectively, these data indicate for the first time a significant role of Mrp2 in biliary elimination of glucuronide and sulfate metabolites of each of the six silymarin flavonolignans.
Table 2.
Apparent Hepatobiliary Clearances of Glucuronide and Sulfate Conjugates and Total (glucuronide+sulfate) Silymarin Flavonolignans Conjugates in WT vs TR- Rats
| Flavonolignan | ClGlucuronide,app (mL/min) | ClSulfate,app (mL/min) | ClTotal conj.,app (mL/min ) | |||
|---|---|---|---|---|---|---|
| WT | TR- | WT | TR- | WT | TR- | |
| SC | 3.00 ± 0.89 | 0.18 ± 0.07* | 2.05 ± 0.78 | 0.53 ± 0.22* | 5.05 ± 1.66 | 0.71 ± 0.27* |
| SD | 9.04 ± 3.57 | 0.59 ± 0.31* | 2.33 ± 0.97 | 0.47 ± 0.09 | 11.37 ± 4.55 | 1.06 ± 0.33* |
| SA | 1.34 ± 0.49 | 0.05 ± 0.01* | 1.50 ± 0.51 | 0.34 ± 0.02* | 2.84 ± 1.00 | 0.40 ± 0.03* |
| SB | 1.21 ± 0.41 | 0.02 ± 0.00* | 4.15 ± 1.52 | 0.68 ± 0.13* | 5.36 ± 1.93 | 0.71 ± 0.13* |
| ISA | 1.39 ± 0.74 | 0.05 ± 0.02 | 7.12 ± 3.24 | 1.59 ± 0.07 | 8.51 ± 3.97 | 1.64 ± 0.09 |
| ISB | 0.99 ± 0.73 | 0.08 ± 0.03 | 5.04 ± 2.64 | 1.66 ± 0.32 | 6.03 ± 3.36 | 1.74 ± 0.34 |
Data are expressed as mean ± S.E.M (n=3). Apparent Hepatobiliary clearance (Clb,app) is calculated as cumulative amount of conjugated flavonolignans excreted in bile (Ae) divided by the respective AUC0→90 min of the parent flavonolignan in the perfusate.
p<0.05, significantly different from WT
Discussion
In the present study, we compared the hepatobiliary disposition of silymarin using isolated perfused livers from WT and TR- rats. The initial perfusate concentration of total silymarin flavonolignans used in this study (26 μg/ml) was within the range of plasma concentrations for total flavonolignans attained in vivo with oral dosing. Peak concentrations of silybin A and B between 0.06 μg/ml with silymarin doses of 200 mg/kg (Morrazoni et al., 1993) and 9.02 μg/ml with silipide doses of 200 mg/kg (Morrazoni et al., 1992, Morrazoni et al., 1993) have been observed in pharmacokinetic studies in rats. Concentrations of silybin A and B as high as 10-40 μg/ml have been observed in clinical studies (Flaig et al., 2007).
High first-pass phase II metabolism is believed to be the major factor responsible for the poor bioavailability of silymarin in man, since glucuronide conjugates account for approximately 90% of the total amount of silybin A and silybin B in blood following oral doses (Gatti and Perucca, 1994; Weyhenmeyer et al., 1992). In rats, glucuronide and sulfate conjugates accounted for 98% of silybin A and silybin B in blood and bile (Morrazoni et al., 1992; Morrazoni et al., 1993). In humans, silymarin conjugates are excreted primarily into bile since less that 5% of the dose is excreted in urine (Weyhenmeyer et al., 1992). In addition, reduced clearance of silybin conjugates, consistent with impaired biliary excretion of conjugates, was observed in patients with extrahepatic biliary obstruction (Schandalik et al., 1992). In our study, all six silymarin flavonolignans were cleared rapidly from the perfusate, and up to 100% of silymarin flavonolignans were excreted as conjugates into bile at the end of the 90-min perfusion. Although between 70-85% of the total dose was recovered in perfusate, bile, and perfused liver, conclusions regarding mass balance in our study cannot be stated with certainty given the high variability observed with IPRL studies. It is possible that formation of additional metabolites may account for a portion of the dose since the silymarin concentration used in our study has been associated with the formation of cytochrome P450-catalyzed demethylated and hydroxylated products in vitro (Jančová et al., 2007; Gunaratna and Zhang, 2003).
While the specific transport proteins involved in the elimination of silymarin and its conjugates have not been identified, previous studies have shown that silymarin has inhibitory effects on the active transport of drugs mediated by Pgp, Mrp1, Mrp4 and Mrp5 (Zhang and Morris, 2003; Nguyen H et al., 2003; Lania-Pietrzak B et al., 2005; Wu CP et al., 2005; Wu et al., 2008). However, there have been no direct investigations of the biliary excretion of silymarin and its conjugates, which could present a complex problem since each of the six major silymarin flavonolignans could have different affinities for the various hepatobiliary transport proteins. Mrp2 is a canalicular organic anion transport protein that is responsible for the biliary elimination of organic anions such as the anticancer drug methotrexate (Luo et al., 2007). Mrp2 also has been shown to be an important transport protein for the biliary excretion of polyphenolic natural products such as quercetin (van Zanden et al., 2007), resveratrol (Maier-Salamon et al., 2008), and 4-methylumbelliferone (Zamek-Gliszczynski et al., 2006b). To establish the dependence of silymarin flavonolignans on Mrp2 for biliary excretion, the present studies were conducted in isolated perfused livers from Mrp2-deficient TR- rats, which are naturally occurring mutants of the Wistar rat (Jansen et al., 1985; König et al., 1999). TR- rats have hereditary conjugated hyperbilirubinemia and exhibit elevated bile acid concentrations, reduced bile flow, up-regulation of basolateral transport protein Mrp3, and display impaired biliary excretion of most organic anions. Livers from TR- rats also exhibit negligible biliary excretion of glucuronide and glutathione conjugates, whereas biliary excretion of sulfate conjugates may only be partially impaired (Maier-Salamon et al., 2008). Our study using isolated perfused livers from TR- rats indicated that Mrp2 is the major canalicular transport protein involved in the biliary excretion silymarin flavonolignan conjugates. Although biliary excretion of the parent silymarin flavonolignans remained unchanged in the TR- and WT IPRLs, the rapid phase II metabolism to glucuronide and sulfate conjugates may limit their availability for active transport. For each parent flavonolignan, < 5% of the dose was recovered in bile as unchanged flavonolignan at the end of each perfusion in both WT and TR- IPRLs, indicating that other canalicular transport proteins such as Pgp or Bcrp may mediate high affinity transport.
The basolateral organic anion transport protein, Mrp3, is upregulated as a compensatory mechanism for the absence of Mrp2 in TR- rats (Ogawa et al., 2000; Johnson et al., 2006.). In TR- rats, increased basolateral efflux of methotrexate (Luo et al., 2007), gemfibrozil (Kim et al., 2003), acetaminophen glucuronide (Xiong et al., 2002) which are Mrp2 substrates, have been observed. During the first 30 minutes of the perfusion when parent flavonolignans were almost completely extracted from perfusate by both TR- and WT IPRLs, the rate of appearance of silybin A and silybin B conjugates in the perfusate was significantly greater for TR- compared to WT IPRLs. While this increase in basolateral efflux of flavonolignan conjugates in TR- rats is consistent with an upregulation of Mrp3, redirection of flavonolignan conjugates from canalicular to basolateral excretory pathways would be expected to occur in TR- rats due to the loss of Mrp2.
Information on the influence of phase II conjugation pathways on the biliary excretion of natural products is limited. Structural rather than stereo differences between the flavonolignans appeared to have the greatest influence on the extent of biliary elimination observed in our study. However, the finding that silydianin was the only silymarin flavonolignan that was primarily glucuronidated and almost completely excreted into bile suggests that flavonolignan glucuronide conjugates may be the preferred substrate for Mrp2. In humans, glucuronidation appears to be a preferred pathway for phase II metabolism of silybin B, silydianin, isosilybin B and silychristin (Wen et al., 2008).
In addition to Mrp2, other canalicular transport proteins may promote the biliary excretion of sulfate conjugates in rats (Zamek-Gliszczynski et al., 2005; Zamek-Gliszczynski et al., 2006b). For example, the biliary excretion of glucuronide conjugates of resveratrol was decreased to a greater extent than the sulfate conjugates in TR- rats (Maier-Salamon et al., 2008). A similar trend was noted in the present study, where biliary excretion of glucuronide conjugates of each flavonolignan was reduced to a greater extent in TR- IPRLs than the biliary excretion of sulfate conjugates.
An important finding from our study was that the conjugates of silydianin were almost exclusively excreted into bile in the WT IPRLs, which suggests that silydianin may have the greatest potential to undergo enterohepatic recycling. In addition, the almost complete recovery of the dose for silydianin as glucuronide conjugates in the bile of WT IPRLs, which was reduced by ∼ 92% in TR- rat livers, suggests that this flavonolignan may be useful as a probe substrate for assessing changes in the functional activity of Mrp2. Many transport proteins of the ABC family such as MRP2 appear to be down-regulated in the livers of patients with chronic HCV (Hinoshita et al, 2001). In addition, while other studies have failed to demonstrate changes in expression detected by immunohistochemical techniques, there appears to be differences in the extent of recycling of canalicular transporters between the canalicular membrane and intracellular pools (Ros et al., 2003) in liver disease. Also, upregulation of basolateral efflux transport proteins such as MRP3, MRP4 and MRP5 has been observed in liver diseases as an adaptation to the reduced expression of MRP2 (Ros et al., 2003; Barnes et al., 2007). Therefore, a specific MRP2 probe substrate would be useful for quantitating the extent of changes in functional activity irrespective of the expression of total MRP2 protein in diseased liver.
Given the results presented here, which indicate a primary role for Mrp2 in the biliary elimination of silymarin flavonolignan conjugates, it is likely that liver disease may be associated with down-regulation of MRP2 in humans since higher plasma concentrations of silymarin conjugates have been observed in patients with liver disease compared to healthy volunteers (Schrieber et al., 2008a). Similar changes in the disposition of silybin A and B have been reported by Wu et al, 2008 utilizing a rat model of cirrhosis where an approximately two-fold increase in plasma AUC for silybin A and B conjugates was correlated with a 50% reduction in the bile to blood exposure ratio for total silybin A and B in cirrhotic rats compared to control. In addition, phase II metabolites of silychristin and silydianin may have greater specificity for Mrp2 since in this study their conjugates were the most extensively excreted into bile among the six major flavonolignans. A recent observation that among the six flavonolignans, enterohepatic recycling was only observed for the conjugates of silychristin and silydianin in patients with chronic HCV (Schrieber et al., 2008b), suggests that these two flavonolignans may be useful as surrogate markers of functional changes in MRP2 activity. In humans, decreased biliary excretion of flavonolignan conjugates may potentially influence the efficacy of silymarin due to down-regulation of MRP2 and reduced enterohepatic recycling and return of parent flavonolignans in portal blood.
In summary, these data indicate a primary role for Mrp2 in the biliary excretion of silymarin flavonolignan conjugates. Hepatic expression of MRP2 may be altered by chronic liver disease. The finding that silydianin is primarily glucuronidated and excreted into bile suggests that it might serve as a specific MRP2 probe substrate for quantitating functional changes in MRP2 activity resulting from liver disease. The extent to which, alterations in MRP2-mediated biliary excretion of silymarin flavonolignans influence the antioxidant or antiviral activity of silymarin warrants investigation.
Acknowledgments
This work was supported in part by the National Institutes of Health (RO1 GM41935 to K.L.R.B) and by Madaus GmbH.
Abbreviations
- SC
silychristin
- SD
silydianin
- SA
silybin A
- SB
silybin B
- ISA
isosilybin A
- ISB
isosilybin B
- HCV
hepatitis C virus
- Mrp
multidrug resistance associated- protein
- Bcrp
breast cancer resistance protein
- WT
Mrp2-competent
- TR-
Mrp2-deficient
- IPRL
isolated perfused rat liver
- LC-ESI-MS
liquid chromatography-electrospray ionization-mass spectrometry
- AUC0→90
area under the plasma concentration-time curve from time 0 to 90 min
References
- Barnes SN, Aleksunes LM, Augustine L, Scheffer GL, Goedken MJ, Jakowski AB, Pruimboom-Brees IM, Cherrington NJ, Manautou JE. Induction of hepatobiliary efflux transporters in acetaminophen-induced acute liver failure cases. Drug Metab Dispos. 2007;35:1963–1969. doi: 10.1124/dmd.107.016170. [DOI] [PubMed] [Google Scholar]
- Bonifaz V, Shan Y, Lambrecht R, Donohue SE, Moschenross D, Bonkoovsky H.Silymarin down-regulates HCV core and up-regulates hemeoxygenase-1 gene in expression in the CNS3 replicon line of human liver cells Hepatology 2007464, Suppl, 1):363A [Google Scholar]
- Brouwer KL, Thurman RG. Isolated Perfused Liver. Pharm Biotechnol. 1996;8:161–192. doi: 10.1007/978-1-4899-1863-5_10. [DOI] [PubMed] [Google Scholar]
- Cermak R, Wolffram S. The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms. Curr Drug Metab. 2006;7:729–744. doi: 10.2174/138920006778520570. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Roma MG. Silymarin as a new hepatoprotective agent in experimental cholestasis: New possibilities for an ancient medication. Curr Med Chem. 2006;13:1055–1074. doi: 10.2174/092986706776360950. [DOI] [PubMed] [Google Scholar]
- Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. A phase I and pharmacokinetic study of silybin-phytosome in prostrate cancer patients. Invest New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- Gatti G, Perucca E. Plasma concentrations of free and conjugated silbin after oral intake of a siybin-phosphatidylcholine complex (silipide) in healthy volunteers. Int J Clin Pharmacol Ther. 1994;32:614–617. [PubMed] [Google Scholar]
- Gores GJ, Kost LJ, LaRusso NF. The isolated perfused rat liver: Conceptual and Practical Considerations. Hepatology. 1986;6:511–517. doi: 10.1002/hep.1840060331. [DOI] [PubMed] [Google Scholar]
- Gunaratna C, Zhang T. Application of liquid chromatography-electrospray ionizationion trap mass spectrometry to investigate the metabolism of silibinin in human liver microsomes. J Chromatogr B. 2003;794:303–310. doi: 10.1016/s1570-0232(03)00484-7. [DOI] [PubMed] [Google Scholar]
- Hinoshita E, Taguchi K, Inokuchi A, Uchiumi T, Kinukawa N, Shimada M, Tsuneyoshi M, Sugimachi K, Kuwano M. Decreased expression of an ATP-binding cassette transporter, MRP2, in human livers with hepatitis virus C infection. J Hepatol. 2001;35:765–773. doi: 10.1016/s0168-8278(01)00216-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann U, Kroemer HK. The ABC transporters MDR1 and MRP2: Multiple functions in disposition of xenobiotics and drug resistance. Drug Metab Rev. 2004;36:669–701. doi: 10.1081/dmr-200033473. [DOI] [PubMed] [Google Scholar]
- Jančová P, Anzenbacherová E, Papoušková B, Lemr K, Lužá P, Veinlichová A, Anzenbacher P, Šimánek V. Silybin is metabolized by cytochrome P450 2C8 in vitro. Drug Metab Dispos. 2007;35:2035–2039. doi: 10.1124/dmd.107.016410. [DOI] [PubMed] [Google Scholar]
- Jansen PL, Peters WH, Lamers WH. Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology. 1985;5:573–579. doi: 10.1002/hep.1840050408. [DOI] [PubMed] [Google Scholar]
- Johnson BM, Zhang P, Schuetz JD, Brouwer KLR. Characterization of transport protein expression in multidrug resistance associated protein (MRP)2-deficient rats. Drug Metab Dispos. 2006;34:556–562. doi: 10.1124/dmd.105.005793. [DOI] [PubMed] [Google Scholar]
- Kim MS, Liu DQ, Strauss JR, Capodanno I, Yao Z, Fenyk-Melody JE, Franklin RB, Vincent SH. Metabolism and disposition of gemfibrozil in wistar and multidrug resistance-associated protein 2-deficient TR-rats. Xenobiotica. 2003;33:1027–1042. doi: 10.1080/00498250310001602720. [DOI] [PubMed] [Google Scholar]
- Kim N-C, Graf TN, Sparacino CM, Wani MC, Wall ME. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum) Org Biomol Chem. 2003;1:1684–1689. doi: 10.1039/b300099k. [DOI] [PubMed] [Google Scholar]
- König J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Lania-Pietrzak B, Michalak K, Hendrich AB, Mosiadz D, Grynkiewicz G, Motohashi N, Shirataki Y. Modulation of MRP1 protein transport by plant, and synthetically modified flavonoids. Life Sciences. 2005;77:1879–1891. doi: 10.1016/j.lfs.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Luo G, Garner CE, Xiong H, Hu H, Richards LE, Brouwer KL, Duan J, Decicco CP, Maduskuie T, Shen H, Lee FW, Gan LS. Effect of DPC 333 [(2R)-2-{(3R)-3-Amino-3-[4-(2-methylquinolin-4-ylmethoxy)phenyl]-2-oxopyrrolidin-1-yl}-N-hydroxy-4-methylpentanamide], a human tumor necrosis factor α converting enzyme inhibitor, on the disposition of methotrexate: a transporter based drug-drug interaction case study. Drug Metab Dispos. 2007;35:835–840. doi: 10.1124/dmd.106.013946. [DOI] [PubMed] [Google Scholar]
- Maier-Salamon A, Hagenaeur B, Reznicek G, Szekeres T, Thalhammer T, Jäqer W. Metabolism and Disposition of resveratrol in the isolated perfused rat liver: Role of Mrp2 in the biliary excretion of glucuronides. J Pharm Sci. 2008;97:1615–1628. doi: 10.1002/jps.21057. [DOI] [PubMed] [Google Scholar]
- Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: a systematic review. J Viral Hepat. 2005;12:559–567. doi: 10.1111/j.1365-2893.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- McDonagh AF, Lightner DA, Nogales DF, Norona WS. Biliary excretion of a stretched bilirubin in UGT1A1-deficient (Gunn) and MRP2 deficient (TR-) rats. FEBS Lett. 2001;506:211–215. doi: 10.1016/s0014-5793(01)02916-7. [DOI] [PubMed] [Google Scholar]
- Morazzoni P, Magistretti MJ, Giachetti C, Zanolo G. Comparative bioavailability of silipide, a new flavanolignan complex, in rats. Eur J Drug Metab Pharmacokinet. 1992;17:39–44. doi: 10.1007/BF03189986. [DOI] [PubMed] [Google Scholar]
- Morazzoni P, Montalbetti A, Malandrino S, Pifferi G. Comparative pharmacokinetics of silipide and silymarin in rats. Eur J Drug Metab Pharmacokinet. 1993;18:289–297. doi: 10.1007/BF03188811. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Zhang S, Morris ME. Effects of flavonoids on MRP1-mediated transport in Panc-1 cells. J Pharm Sci. 2003;92:250–257. doi: 10.1002/jps.10283. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Suzuki H, Hirohashi T, Toshihisa I, Meier PJ, Hirose K, Akizawa T, Yoshioka M, Sugiyama Y. Characterization of inducible nature of MRP3 in rat liver. Am. J. Physiol. Gastrointest Liver Physiol. 2000;278:G438–G446. doi: 10.1152/ajpgi.2000.278.3.G438. [DOI] [PubMed] [Google Scholar]
- Pauluama CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytqat GN, Borst P, Baas F, Oude Elferink RP. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25:1539–1542. doi: 10.1002/hep.510250635. [DOI] [PubMed] [Google Scholar]
- Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized silymarin. Gastroenterology. 2007;132:1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Ros JE, Libbrecht L, Geuken M, Jansen PL, Roskams TA. High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe liver disease. J Pathol. 2003;200:553–560. doi: 10.1002/path.1379. [DOI] [PubMed] [Google Scholar]
- Schandalik R, Gatti G, Perucca E. Pharmacokinetics of silybin in bile flowing administration of silipide and silymarin in cholecystectomy patients. Drug Res. 1992;42:964–968. [PubMed] [Google Scholar]
- Schrieber SJ, Wen Z, Dumas TE, Vourvahis M, Smith PC, Kashuba AD, Fried MW, Hawke RL. The pharmacokinetics of silymarin is altered in patients with chronic hepatitis c virus and nonalcoholic fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos. 2008a;36:1909–1916. doi: 10.1124/dmd.107.019604. [DOI] [PubMed] [Google Scholar]
- Schrieber SJ, Soule TA, Wen Z, Wahed AS, Smith PC, Fried MW, Reddy RK, Navarro VJ, Afdhal N, Belle SH, Berman J, Liu Q-Y, Doo E, Hawke RL. Phase I study to evaluate the safety, tolerability, and pharmacokinetics of silymarin following chronic dosing in patients with chronic hepatitis C. Gastroenterology. 2008b;S134:P435396. [Google Scholar]
- Seeff LN, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, Lindsay KL, Lok ASF, Di Bisceglie AM, Lee WM, Ghany MG, HALT-C Trial Group Herbal product use by persons enrolled in the hepatitis C antiviral long-term treatment against cirrhosis (HALT-C) trial. Hepatology. 2008;47:605–612. doi: 10.1002/hep.22044. [DOI] [PubMed] [Google Scholar]
- van Zanden JJ, van der Woude H, Vaessen J, Usta M, Wortelboer HM, Cnubben NH, Rietjens IM. The effect of quercetin Phase II metabolism on its MRP1 and MRP2 inhibiting potential. Biochem Pharmacol. 2007;74:345–351. doi: 10.1016/j.bcp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. Pharmacokinetics and metabolic profile of free, conjugated and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos. 2008;36:65–72. doi: 10.1124/dmd.107.017566. [DOI] [PubMed] [Google Scholar]
- Weyhenmeyer R, Mascher H, Birkmayer J. Study on dose-linearity of the pharmacokinetics of silibinin diastereomers using a new stereospecific assay. Int J Clin Pharmacol Ther Toxicol. 1992;30:134–138. [PubMed] [Google Scholar]
- Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5) FEBS J. 2005;272:4725–4740. doi: 10.1111/j.1742-4658.2005.04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JW, Lin LC, Hung SC, Lin CH, Chi CW, Tsai TH. Hepatobiliary Excretion of silibinin in normal and liver cirrhotic rats. Drug Metab Dispos. 2008;36:589–596. doi: 10.1124/dmd.107.017004. [DOI] [PubMed] [Google Scholar]
- Xiong H, Suzuki H, Sugiyama Y, Meier PJ, Pollack GM, Brouwer KLR. Mechanisms of impaired biliary excretion of acetaminophen glucuronide after acute Phenobarbital treatment or Phenobarbital pretreatment. Drug Metab Dispos. 2002;30:962–969. doi: 10.1124/dmd.30.9.962. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Hoffmaster KA, Tian X, Zhao R, Polli JW, Humphreys JE, Webster LO, Bridges AS, Kalvass JC, Brouwer KL. Multiple mechanisms are involved in the biliary excretion of acetaminophen sulfate in the rat: Role of MRP2 and BCRP1. Drug Metab Dispos. 2005;33:1158–1165. doi: 10.1124/dmd.104.002188. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Hoffmaster KA, Nezasa K, Tallman MN, Brouwer KL. Integration of hepatic drug transporters and phase II metabolizing enzymes: mechanisms of hepatic excretion of sulfate, glucuronide, and glutathione metabolites. Eur J Pharm Sci. 2006a;27:447–486. doi: 10.1016/j.ejps.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Hoffmaster KA, Humphreys JE, Tian X, Nezasa K, Brouwer KL. Differential involvement of Mrp2 (Abcc2) and Bcrp (Abcg2) in biliary excretion of 4-methylumbelliferyl glucuronide and sulfate in the rat. J Pharmacol Exp Ther. 2006b;319:459–467. doi: 10.1124/jpet.106.101840. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang X, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004;21:1263–1273. doi: 10.1023/b:pham.0000033015.84146.4c. [DOI] [PubMed] [Google Scholar]
- Zhang S, Morris ME. Effect of the flavonoids biochanin A and silymarin on the P-glycoprotein-mediated transport of digoxin and vinblastine in human intestinal Caco-2 cells. Pharm Res. 2003;20:1184–1191. doi: 10.1023/a:1025044913766. [DOI] [PubMed] [Google Scholar]