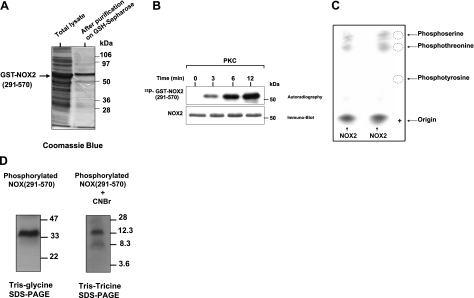
Figure 5.
Phosphorylation of recombinant gp91phox/NOX2 cytosolic flavoprotein domain by purified PKC in vitro and phosphoamino acid analysis. A) Recombinant NOX2 (291-570) was expressed in bacteria, and purified protein was analyzed by SDS-PAGE and Coomassie blue staining as described. B) Recombinant protein was incubated with [γ-32P]-ATP in the presence of PKC, reactions were terminated at the indicated times, and proteins were separated by SDS-PAGE and analyzed by autoradiography and immunoblotting with the anti-NOX2 antibodies. C) Phosphorylated NOX2 was separated by SDS-PAGE, tranferred to PVDF, excised, and subjected to acid hydrolysis, as described. Phosphoamino acids were collected and mixed with or without standard markers (phosphoserine, phosphothreonine, phosphotyrosine) and separated by thin layer electrophoresis. Standard phosphoamino acids were visualized by 0.2% ninhydrin, and phosphorylated amino acids were detected by autoradiography. Duplicate samples are shown. D) Phosphorylated NOX2 (291-570) was separated by SDS-PAGE, tranferred to nitrocellulose, excised, and then incubated with 50 mg/ml CNBr in 70% (v/v) formic acid for 16 h at room temperature in the dark. Supernatant was dried in a speed-vac and lyophilized. Peptides were analyzed by Tris-Tricine SDS-PAGE followed by autoradiography. Data are representative of 4 experiments.