Summary of recent advances
Sex in vertebrates is determined by genetic- or environmentally-based signals. These signals initiate molecular cascades and cell-cell interactions within the gonad that lead to the adoption of the male or female fate. Previously, genetic- and environmentally-based mechanisms were thought to be distinct, but this idea is fading as a result of the unexpected discovery of coincident genetic and thermal influences within single species. Together with accumulating phylogenetic evidence of frequent transitions between sex-determining mechanisms, these findings suggest that genetic and environmental sex determination actually represent points on a continuum rather than discrete categories, and that populations may shift in one direction or the other in response to mutations or changing ecological conditions. Elucidation of the underlying molecular basis of sex determination in mice has yielded a bistable model of mutually antagonistic signaling pathways and feedback regulatory loops. This system would be highly responsive to changes in the upstream primary signal and may provide a basis for the rapid evolution of and transitions between different methods of sex determination.
Introduction
Across vertebrates, primary sex determination is defined as the decision within the bipotential gonad to develop as a testis or an ovary. Within the past decade, the traditional view of this process, in which a strict division is drawn between species that employ genetic mechanisms (genetic sex determination, GSD; e.g., mammals, birds) to determine sex and those that use environmental mechanisms (such as temperature-dependent sex determination, TSD; e.g., crocodiles), has been supplanted by the theory that GSD and TSD actually represent points on a continuum along which populations can and do shift, under selective pressure [1,2,3••]. This novel perspective is strengthened by the recent accumulation of empirical data suggesting that genetic elements influence systems that use TSD, and that functional or vestigial temperature sensitivity is present in organisms that employ GSD, even some with heteromorphic sex chromosomes [4•,5•,6•,7••]. Additionally, many of the cellular processes, transcription factors and signaling pathways involved in sex determination and gonadogenesis are conserved across vertebrates, implying that the underlying machinery may be similar despite modifications in the dominant upstream signal used. This perspective will be discussed in the present review, in the context of converging ideas about how sex-specific development of the gonads is initiated.
Rapid evolutionary transitions between sex-determining mechanisms
The methods of sex determination (e.g., GSD (XX/XY, ZZ/ZW, or homomorphy), TSD, polygenic, or density-dependent) used by many species of reptile, amphibian and fish have been elucidated in recent years (Figure 1)[3••,8-10]. When these are plotted onto a phylogenetic map, the evolutionary lability of sex determination is apparent within several major branches of the tree, where numerous transitions must have occurred to achieve the present diversity [3••]. Most of these inferred transitions remain unexplained, but two studies provide definitive examples of rapid and recent transitions between different GSD mechanisms.
Figure 1.
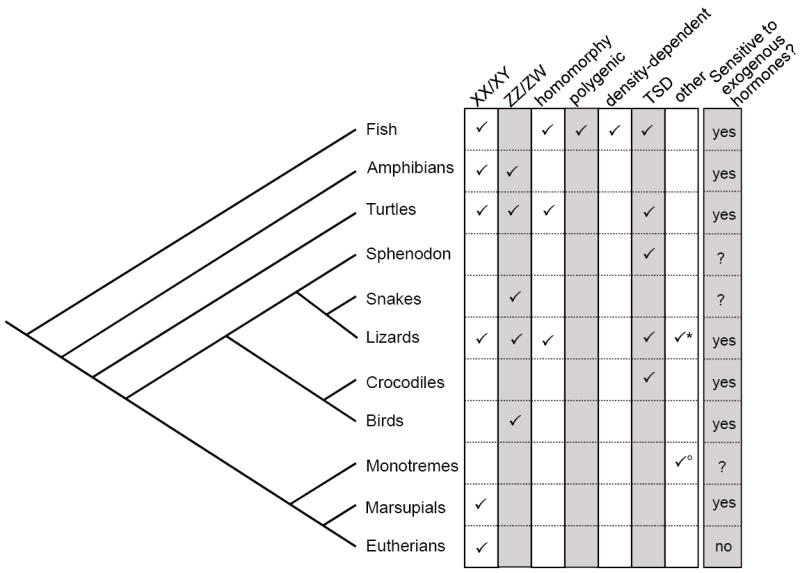
Sex determination in extant vertebrates. Fish, amphibians, turtles, and lizards each exhibit more than one method of sex determination, which fall into genetic (GSD) and environmentally based categories. XX/XY and ZZ/ZW refer to male and female heterogametic systems, respectively, while homomorphy refers to GSD in the absence of differentiated sex chromosomes. Polygenic and density-dependent sex determination are characteristic of a subset of fish, and many reptiles and fish determine sex according to the incubation temperature of the egg (TSD). With the exception of eutherian mammals, most vertebrate embryos are susceptible to exogenous hormone-induced sex reversal. *Co-occurrence of TSD and GSD has been noted in several species of lizards. °Monotremes (i.e., platypus and echidna) have a complex arrangement of X and Y sex chromosomes, which assemble into a chain during meiosis.
Investigation of a species of Japanese frog, Rana rugosa, demonstrated the presence of both ZZ/ZW and XX/XY heterogamety in neighboring populations, with homology between the Z-Y and W-X chromosomes. This evidence suggests that population intermixing may have driven the transitions between chromosomal complements in this case [11•].
In two closely related medaka species, a master male sex-determining gene, DMY, derived from the conserved male-promoting gene DMRT1, was identified on the Y chromosome [12,13]. However, this gene is not present in the genome of the other three species of this genus, which must, therefore, use another method to determine sex [14,15]. In a relationship akin to that of murine Sry and Sox9 (discussed below), DMY appears to preempt the female pathway by advancing expression of a DMRT1 ortholog in the XY medaka gonad, thus promoting testis development. This finding implies that gene duplication can initiate rapid transitions to new forms of GSD.
Overriding GSD by hormones and temperature
Eutherian mammals, with XX/XY male heterogamety, comprise one of the most strictly GSD groups in the animal kingdom. Classic work in the mouse and human systems demonstrated that expression of the Y-chromosome-linked Sry gene in the supporting cell lineage leads to their differentiation as Sertoli cells and the adoption of the testis fate [16,17]. In the absence of this upstream signal, ovarian development ensues. The evolution of both viviparity and endothermy in eutherian mammals required a mechanism not based on temperature or environment. The accumulation of fertility factors on the Y chromosome and the low viability of XY oocytes [18,19] operate to fix the GSD mechanism in mammals. However, viviparity and TSD coexist in several reptiles where pregnant females can influence offspring sex ratio through their thermoregulatory behavior [20,21].
In contrast to eutherian mammals, sex determination in other vertebrate species that employ GSD is sensitive to exogenous hormones (Figure 1). Both marsupials, which share the Sry-dependent XX/XY system of eutherian mammals [22,23] (though sex determination occurs after birth [24]), and birds, which exhibit ZZ/ZW female heterogamety with an unknown upstream signal [25], are sensitive to the application of hormones [8,9]. Susceptibility to hormone treatment is shared by most reptiles, including numerous species traditionally classified as either GSD or TSD [26-28].
Sex in several reptilian GSD species is also affected by incubation at temperatures toward the limit of the viable range. A species of scincid lizard, Bassiana duperreyi, with heteromorphic sex chromosomes (XX/XY) shows both GSD and TSD mechanisms operating within a single population [5•,29]. Incubation at a low temperature can sex-reverse this lizard from female to male [29]. While incubation temperature has been previously shown to override genetic sex in several amphibian and fish species [30-32], this is the first report of a sensitive heterogametic species. In this system, the observed unidirectional temperature-induced sex reversal (female to male) would produce XX males, not XY females. Fitness-compromised YY individuals will therefore not arise in the next generation, and both mechanisms might be simultaneously maintained without significant risk.
Similarly, high egg incubation temperatures can also induce discordant sexual phenotypes in a ZZ/ZW agamid lizard, Pogona vitticeps [4•]. Here, the converse argument applies. Sex reversal in this female heterogametic system also appears to be functionally unidirectional (male to female) yielding ZZ females rather than ZW males. Potentially disadvantaged individuals with a WW genotype will not be generated. Together, these studies suggest that limited thermal sensitivity within a GSD system may have adaptive significance (see below), or serve as raw material for an evolutionary transition to TSD under a new set of selective pressures.
Thermosensitive gene expression in a GSD turtle
In TSD species, many genes known to be involved in sex determination or gonadogenesis show temperature-specific expression patterns during the temperature-sensitive period of development, before sex has been determined; however, the functional significance of these findings is not clear [e.g. 33,34,35•,36-38]. For example, expression of DMRT1 in the red-eared slider turtle, Trachemys scripta, is elevated at the male-producing temperature as compared to the higher female-producing temperature at stages when sex is still labile [39]. Gonadal expression levels of the WT1 gene were compared between related TSD and GSD turtle species to determine whether any vestigial thermal sensitivity is present in the gene regulatory network underlying gonad development in the GSD species [6•]. When incubated at the temperature that produces 100% males in the TSD species, gonads of both species exhibited higher WT1 levels. However, artificially elevated WT1 levels did not override the genetic mechanism in the GSD species and induce sex reversal [6•]. Nonetheless, this study illustrates the subtle thermal sensitivity that may exist within the underlying genetic network. In these systems, a single mutation leading to thermosenstivity of one or more key genes could modify a genetic mechanism and initiate a transition from GSD towards TSD (Figure 2).
Figure 2.
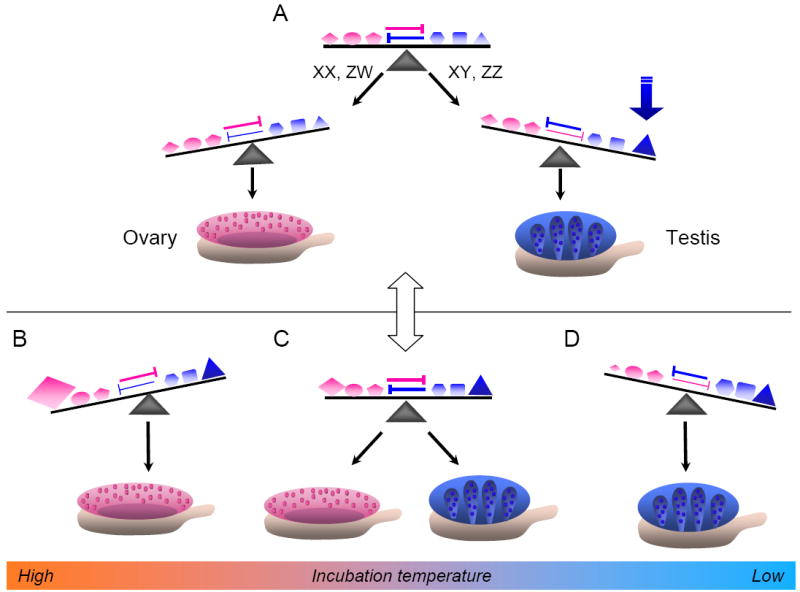
Hypothetical evolutionary transition between GSD and TSD systems. A, Based on the mammalian model, the bipotential gonad is initially balanced between alternative fates by mutually antagonistic male and female factors. The appearance of a segregating, dominant allele of a key gene(s) in either the male or female pathway can fix a genetic mechanism. This may occur by gene duplication and evolution of a sex chromosome (as in the case of Sry in mammals or Dmy in medaka). B, Subsequent acquisition of a temperature-sensitive mutation in a female gene (e.g., aromatase) that increases expression or activity at a high temperature, may override the male factor and shift the balance towards the female pathway. C, At intermediate temperatures, the pathways will remain balanced, so that males and females are produced stochastically at equal frequencies. D, At a low temperature, the mutated female factor will be far from its thermal optimum, activity will drop, and the balance will shift towards the male pathway. Depending on the strength of the determining factor, the system may be more or less stable to perturbation by the appearance of new mutations. Selective pressure may favor changes that maintain a balanced sex ratio.
Geographic pressures on TSD
The pivotal temperature (TP) for a TSD species is defined as the range of incubation temperatures that produce a 1:1 male to female sex ratio. In many species, temperatures below the TP will yield an increasingly male-biased ratio, and higher temperatures will generate more females (MF), though this pattern is often reversed (FM). While only one TP has been observed in many species, others exhibit a FMF pattern with two distinct transition points [7••]. It has been proposed that the existence of a single TP may reflect viability constraints at one end of the temperature range, rather than a fundamental difference between the underlying temperature-sensitive mechanisms [4•]. The TP(s) for a given species has been shown to shift according to the local climate and latitude of individual populations, suggesting the presence of selective pressure to maintain a balanced sex ratio and inherent flexibility in the thermosensitivity of the system [7].
Evolutionary advantage of TSD
As TSD has been documented in a wide array of species, it must carry selective advantages in particular environments. Unfortunately, the longevity and delayed sexual maturity characteristic of most reptilian TSD species have made direct evaluations of reproductive fitness impractical. The Charnov-Bull model predicts that TSD should be favored if the fitness of (either or both) males and females is enhanced by development under a particular set of environmental conditions [40]. In their study of the TSD agamid lizard Amphibolurus muricatus, Warner and Shine have provided the first empirical evidence in reptiles that supports this model [41••]. This species of lizard matures rapidly, allowing its reproductive output to be tracked in the laboratory and genetically confirmed. Females are produced at both high and low temperatures, while intermediate temperatures yield a 1:1 male to female ratio. The authors generated males at three representative temperatures by treating developing embryos with a hormone inhibitor, and then compared the lifetime fecundity of male individuals produced at the natural (intermediate) male-producing temperature to that of males from temperatures that normally produce only females. Females generated at the intermediate temperature were also compared to females from eggs incubated at high and low temperatures. The results show that the fecundity of each sex was maximized by development at the temperature that naturally produces that sex. For example, males incubated at the intermediate temperature had higher reproductive outputs than sex-reversed individuals from female-producing temperatures, in accord with the predictions of the Charnov-Bull model.
Antagonistic pathways: a plastic system for evolutionary adaptation
The decision to develop as a male or female depends on whether the gonad develops as a testis or an ovary. In mammals, this decision rests on the fate of the supporting cell lineage, which either initiates differentiation as Sertoli (male) or follicle (female) cells. Fgf9 and Wnt4 act as mutually antagonistic signals that converge on Sox9 during sex determination to regulate the fate of the supporting cell lineage in mice [42•]. Both Fgf9 and Wnt4 are expressed in the bipotential gonad. Sry triggers the up-regulation of Sox9, which then up-regulates Fgf9 in a feed forward loop that is required to sustain Sox9 expression, establish Sertoli cell differentiation and repress Wnt4. In the absence of Fgf9, or its receptor, FGFR2 [43,44], Sox9 is down-regulated. Wnt4 gains control of the fate of gonadal cells and initiates follicle cell (ovary) development. Surprisingly, in Wnt4 mutant XX gonads, Sox9 expression is transiently elevated in a manner reminiscent of the initiation of the male pathway, and although sex-reversal is incomplete, gonads undergo morphological changes characteristic of testis development [42•]. Importantly, this occurs in XX gonads that lack Sry. Conversely, although Sry is the dominant switch in mammals, it is now clear that the male pathway can be over-ridden by β-catenin expression in XY gonads [45]. Both these and other lines of evidence [46-49] argue strongly for regulation of sex determination through antagonistic signaling pathways in mammals.
So far, evidence for this model has been demonstrated only in mice and humans. However, mechanisms of sex determination based on antagonistic signaling could be highly labile in evolutionary terms, and might easily be regulated in diverse ways. For example, minor shifts in the timing, level, or activity of a single factor could trigger an imbalance in the system (Figure 2). In some cases a factor with a major influence may have evolved (i.e., DMY in medaka), whereas in others, several factors may have a cumulative influence on the outcome over a relatively long bipotential period. In either case, the canalizing effect of feedback regulation, both within and between cells, acts to stabilize one pathway and force commitment to either testis or ovary fate.
Although both testis/ovary morphology and expression of many of the genes (other than Sry itself) involved in sex determination in mammals are conserved in all vertebrates tested to date [e.g. 35•,50-52], the order of morphological events and the sequence and timing of gene expression are not fully conserved. This suggests that there are multiple entry points into the regulatory loops that establish testis or ovary development (Figure 3). While it is clear that the supporting cell lineage is the cell type where primary commitment occurs in mammals, this is not clear for other vertebrates, where germ cells or steroidogenic precursors may be the focal point of the sex-determining decision. Nonetheless, consideration of the diversity and rapid variation of sex determination systems in the context of this model may be instructive.
Figure 3.
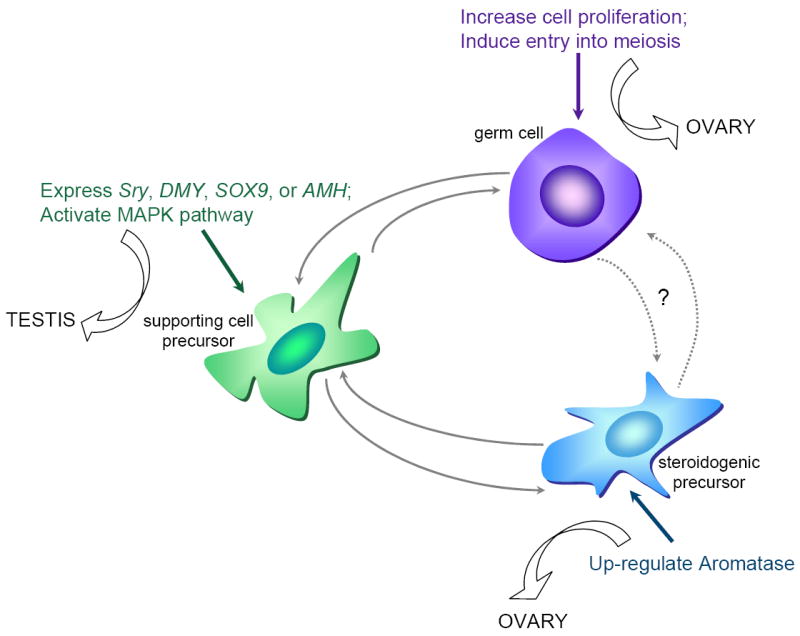
The decision to develop as a testis or ovary may originate in distinct gonad cell types in different vertebrates. For example, Sry is expressed in mammalian supporting cell precursors, leading to their differentiation as Sertoli cells, which then direct germ cells to enter mitotic arrest and steroidogenic precursors to differentiate as Leydig cells [17]. This cascade of cell-cell interactions and differentiation might also be initiated by expression of other male factors in the supporting cell precursors [12,43,53,54]. Alternatively, in other vertebrates, the decision might be made in other cell lineages. Upregulation of aromatase, a steroidogenic enzyme that converts testosterone to estrogen, in the steroidogenic precursors may allow these cells to induce ovarian development by a hormonal mechanism [reviewed in 55]. Similarly, mitotic proliferation of germ cells or entry into meiosis [56] could induce changes in the steroidogenic and supporting cell precursors that together initiate adoption of the female fate. Solid and dotted arrows represent demonstrated and putative cell-cell interactions, respectively.
Conclusions
In contrast to the high conservation of most developmental pathways across species from Drosophila to man, a stunning variety of mechanisms of sex determination exist within the animal kingdom. The distinction between genetic- and environmentally-based primary signals is beginning to blur as a result of the discovery of coincident thermal and genetic influences within single species, as well as the high degree of conservation in the genes governing gonadogenesis. The model of antagonistic signaling and feedback reinforcement that has emerged in mammals provides a possible explanation for the rapid evolution of and transitions between different sex determination mechanisms seen among vertebrates. Although the entry points into and the order of the pathways governing sex determination seem to be different, this general model may be valuable in the quest to understand these highly diverse processes. Surveys for novel differentially expressed genes specific to the avian, reptilian, amphibian, or fish systems, as well as development of methods to test the functional involvement of these genes, will be important to make significant progress toward understanding the molecular basis of sex determination in non-mammalian species.
Acknowledgments
We thank Steve Munger and Leo DiNapoli for insightful comments and critical reading of the manuscript. Funding in the Capel laboratory is provided by the National Science Foundation (0317234) and the National Institutes of Health (HL63054 and HD39963).
Abbreviations
- GSD
genetic sex determination
- TSD
temperature-dependent sex determination
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lindsey A. Barske, Email: lindsey.barske@duke.edu.
Blanche Capel, Email: b.capel@cellbio.duke.edu.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Sarre SD, Georges A, Quinn A. The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. Bioessays. 2004;26:639–645. doi: 10.1002/bies.20050. [DOI] [PubMed] [Google Scholar]
- 2.Bull J. Sex determination: Are two mechanisms better than one? Journal of Bioscience. 2008;32:5–8. doi: 10.1007/s12038-008-0016-9. [DOI] [PubMed] [Google Scholar]
- ••3.Janzen FJ, Phillips PC. Exploring the evolution of environmental sex determination, especially in reptiles. J Evol Biol. 2006;19:1775–1784. doi: 10.1111/j.1420-9101.2006.01138.x. This excellent review discusses the evolutionary history of TSD in reptiles, highlighting the many transitions between sex-determining mechanisms that have occurred in this lineage as well as avenues for future research into the adaptive significance of the TSD strategy.
- •4.Quinn AE, Georges A, Sarre SD, Guarino F, Ezaz T, Graves JA. Temperature sex reversal implies sex gene dosage in a reptile. Science. 2007;316:411. doi: 10.1126/science.1135925. This paper reports temperature-induced sex reversal in a lizard with ZZ/ZW heteromorphy. A threshold model of how the male pathway may be activated or inhibited at different egg incubation temperatures is discussed.
- •5.Radder RS, Quinn AE, Georges A, Sarre SD, Shine R. Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biol Lett. 2008;4:176–178. doi: 10.1098/rsbl.2007.0583. This paper reports the simultaneous presence of TSD and GSD in a species of lizard, such that genotypically female animals present a male phenotype when embryos are incubated under cool temperature regimes.
- •6.Valenzuela N. Relic thermosensitive gene expression in a turtle with genotypic sex determination. Evolution Int J Org Evolution. 2008;62:234–240. doi: 10.1111/j.1558-5646.2007.00279.x. This paper reports unexpected thermal sensitivity in the expression of a gene thought to be required for gonadogenesis in a GSD turtle.
- ••7.Ewert MA, Lang JW, Nelson CE. Geographic variation in the pattern of temperature-dependent sex determination in the American snapping turtle (Chelydra serpentina) Journal of Zoology. 2005;265:81–95. This very interesting study explains the perplexing preexisting data on the direction that pivotal temperatures usually shift across the latitudinal range of a TSD species. The authors also discuss geographic patterns of maternal nest site selection, as well as how these behaviors likely signify a tradeoff between embryo viability and developmental rate.
- 8.Ryuzaki M, Hanada H, Okumoto H, Takizawa N, Nishioka M. Evidence for heteromorphic sex chromosomes in males of Rana tagoi and Rana sakuraii in Nishitama district of Tokyo (Anura: Ranidae) Chromosome Res. 1999;7:31–42. doi: 10.1023/a:1009271110980. [DOI] [PubMed] [Google Scholar]
- 9.Baroiller JF, D’Cotta H. Environment and sex determination in farmed fish. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:399–409. doi: 10.1016/s1532-0456(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 10.Ogata M, Ohtani H, Igarashi T, Hasegawa Y, Ichikawa Y, Miura I. Change of the heterogametic sex from male to female in the frog. Genetics. 2003;164:613–620. doi: 10.1093/genetics/164.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •11.Ogata M, Hasegawa Y, Ohtani H, Mineyama M, Miura I. The ZZ/ZW sex-determining mechanism originated twice and independently during evolution of the frog, Rana rugosa. Heredity. 2008;100:92–99. doi: 10.1038/sj.hdy.6801068. The authors investigated the heterogametic status (XX/XY or ZZ/ZW) of neighboring populations of a Japanese frog, and after elucidating the phylogenetic relationships between these populations, determined that transitions between male and female heterogamety must have occurred on two independent occasions.
- 12.Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda M, Sato T, Toyazaki Y, Nagahama Y, Hamaguchi S, Sakaizumi M. Oryzias curvinotus has DMY, a gene that is required for male development in the medaka, O. latipes. Zoolog Sci. 2003;20:159–161. doi: 10.2108/zsj.20.159. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M. Sex determination in the teleost medaka, Oryzias latipes. Annu Rev Genet. 2005;39:293–307. doi: 10.1146/annurev.genet.39.110304.095800. [DOI] [PubMed] [Google Scholar]
- 15.Kondo M, Nanda I, Hornung U, Asakawa S, Shimizu N, Mitani H, Schmid M, Shima A, Schartl M. Absence of the candidate male sex-determining gene dmrt1b(Y) of medaka from other fish species. Curr Biol. 2003;13:416–420. doi: 10.1016/s0960-9822(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 16.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 17.Lovell-Badge R, Hacker A. The molecular genetics of Sry and its role in mammalian sex determination. Philos Trans R Soc Lond B Biol Sci. 1995;350:205–214. doi: 10.1098/rstb.1995.0153. [DOI] [PubMed] [Google Scholar]
- 18.Alton M, Lau MP, Villemure M, Taketo T. The behavior of the X- and Y-chromosomes in the oocyte during meiotic prophase in the B6.Y(TIR)sex-reversed mouse ovary. Reproduction. 2008;135:241–252. doi: 10.1530/REP-07-0383. [DOI] [PubMed] [Google Scholar]
- 19.Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science. 1997;278:675–680. doi: 10.1126/science.278.5338.675. [DOI] [PubMed] [Google Scholar]
- 20.Wapstra E, Olsson M, Shine R, Edwards A, Swain R, Joss JM. Maternal basking behaviour determines offspring sex in a viviparous reptile. Proc Biol Sci. 2004;271(Suppl 4):S230–232. doi: 10.1098/rsbl.2003.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert KA, Thompson MB. Sex determination. Viviparous lizard selects sex of embryos. Nature. 2001;412:698–699. doi: 10.1038/35089135. [DOI] [PubMed] [Google Scholar]
- 22.Foster JW, Brennan FE, Hampikian GK, Goodfellow PN, Sinclair AH, Lovell-Badge R, Selwood L, Renfree MB, Cooper DW, Graves JA. Evolution of sex determination and the Y chromosome: SRY-related sequences in marsupials. Nature. 1992;359:531–533. doi: 10.1038/359531a0. [DOI] [PubMed] [Google Scholar]
- 23.Sharman GB, Robinson ES, Walton SM, Berger PJ. Sex chromosomes and reproductive anatomy of some intersexual marsupials. J Reprod Fert. 1970;21:57–68. doi: 10.1530/jrf.0.0210057. [DOI] [PubMed] [Google Scholar]
- 24.Coveney D, Shaw G, Renfree MB. Estrogen-induced gonadal sex reversal in the tammar wallaby. Biol Reprod. 2001;65:613–621. doi: 10.1095/biolreprod65.2.613. [DOI] [PubMed] [Google Scholar]
- •25.Smith CA. Sex determination in birds: HINTs from the W sex chromosome? Sex Dev. 2007;1:279–285. doi: 10.1159/000108934. This review discusses the unsettled state of the avian sex determination field, focusing primarily on a candidate sex-determining gene from the W chromosome.
- 26.Bull JJ, Gutzke WH, Crews D. Sex reversal by estradiol in three reptilian orders. Gen Comp Endocrinol. 1988;70:425–428. doi: 10.1016/0016-6480(88)90117-7. [DOI] [PubMed] [Google Scholar]
- 27.Pieau C. Effects of estradiol on the genital apparatus of the embryo of the Mauresque turtle (Testudo graceca L.) Arch Anat Microsc Morphol Exp. 1970;59:295–318. [PubMed] [Google Scholar]
- 28.Pieau C, Girondot M, Richardmercier N, Desvages G, Dorizzi M, Zaborski P. Temperature Sensitivity of Sexual-Differentiation of Gonads in the European Pond Turtle - Hormonal Involvement. Journal of Experimental Zoology. 1994;270:86–94. [Google Scholar]
- 29.Shine R, Elphick MJ, Donnellan S. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecology Letters. 2002;5:486–489. [Google Scholar]
- 30.Baroiller JF, Chourrout D, Fostier A, Jalabert B. Temperature and Sex-Chromosomes Govern Sex-Ratios of the Mouthbrooding Cichlid Fish Oreochromis-Niloticus. Journal of Experimental Zoology. 1995;273:216–223. [Google Scholar]
- 31.Dorazi R, Chesnel A, Dournon C. Opposite Sex Determination of Gonads in 2 Pleurodeles Species May Be Due to a Temperature-Dependent Inactivation of Sex-Chromosomes. Journal of Heredity. 1995;86:28–31. [Google Scholar]
- 32.Chardard D, Penrad-Mobayed M, Chesnel A, Pieau C, Dournon C. Thermal Sex Reversals in Amphibians. In: Valenzuela N, editor. Temperature-Dependent Sex Determination in Vertebrates. Lance VA: Smithsonian Books; 2004. pp. 59–67. [Google Scholar]
- 33.Fleming A, Wibbels T, Skipper JK, Crews D. Developmental expression of steroidogenic factor 1 in a turtle with temperature-dependent sex determination. Gen Comp Endocrinol. 1999;116:336–346. doi: 10.1006/gcen.1999.7360. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey M, Shoemaker C, Crews D. Gonadal expression of Sf1 and aromatase during sex determination in the red-eared slider turtle (Trachemys scripta), a reptile with temperature-dependent sex determination. Differentiation. 2007 doi: 10.1111/j.1432-0436.2007.00182.x. [DOI] [PubMed] [Google Scholar]
- •35.Shoemaker CM, Queen J, Crews D. Response of candidate sex-determining genes to changes in temperature reveals their involvement in the molecular network underlying temperature-dependent sex determination. Mol Endocrinol. 2007;21:2750–2763. doi: 10.1210/me.2007-0263. This paper presents gonad expression profiles of several genes that may be involved in turtle sex determination, and investigates how the expression levels of these genes respond to temperature shifts sufficient to induce sex reversal.
- 36.Spotila LD, Spotila JR, Hall SE. Sequence and expression analysis of WT1 and Sox9 in the red-eared slider turtle, Trachemys scripta. J Exp Zool. 1998;281:417–427. doi: 10.1002/(sici)1097-010x(19980801)281:5<417::aid-jez7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 37.Torres Maldonado LC, Landa Piedra A, Moreno Mendoza N, Marmolejo Valencia A, Meza Martinez A, Merchant Larios H. Expression profiles of Dax1, Dmrt1, and Sox9 during temperature sex determination in gonads of the sea turtle Lepidochelys olivacea. Gen Comp Endocrinol. 2002;129:20–26. doi: 10.1016/s0016-6480(02)00511-7. [DOI] [PubMed] [Google Scholar]
- 38.Rhen T, Metzger K, Schroeder A, Woodward R. Expression of putative sex-determining genes during the thermosensitive period of gonad development in the snapping turtle, Chelydra serpentina. Sex Dev. 2007;1:255–270. doi: 10.1159/000104775. [DOI] [PubMed] [Google Scholar]
- 39.Shoemaker C, Ramsey M, Queen J, Crews D. Expression of Sox9, Mis, and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Dev Dyn. 2007;236:1055–1063. doi: 10.1002/dvdy.21096. [DOI] [PubMed] [Google Scholar]
- 40.Charnov EL, Bull J. When is sex environmentally determined? Nature. 1977;266:828–830. doi: 10.1038/266828a0. [DOI] [PubMed] [Google Scholar]
- ••41.Warner DA, Shine R. The adaptive significance of temperature-dependent sex determination in a reptile. Nature. 2008;451:566–568. doi: 10.1038/nature06519. This paper presents the first conclusive test of the Charnov-Bull model in a reptilian system. The authors show that reproductive fitness of both males and females was enhanced by development at temperatures that naturally produce that sex, as compared to temperatures that normally produce the opposite sex.
- •42.Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. This study presents evidence for a system of mutual antagonism between Fgf9/Sox9 and Wnt4 that balances the mammalian bipotential gonad between the male and female fates.
- 43.Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Capel B. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci U S A. 2007;104:16558–16563. doi: 10.1073/pnas.0702581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagheri-Fam S, Sim H, Bernard P, Jayakody I, Taketo MM, Scherer G, Harley VR. Loss of Fgfr2 leads to partial XY sex reversal. Dev Biol. 2008;314:71–83. doi: 10.1016/j.ydbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Maatouk DM, DiNapoli L, Alvers A, Parker K, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Human Molecular Genetics. 2008 doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- 47.Parma P, Radi O, Vidal VI, Chaboissier MC, Dellambra E, Valentini S, Fuerra L, Schedl A, Camerino G. R-spondin1 plays an essential role in sex determination, skin differentiation and malignancy. Nature Genetics. 2006 doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- 48.Tomaselli S, Megiorni F, De Bernardo C, Felici A, Marrocco G, Maggiulli G, Grammatico B, Remotti D, Saccucci P, Valentini F, et al. Syndromic true hermaphroditism due to an R-spondin1 (RSPO1) homozygous mutation. Hum Mutat. 2008;29:220–226. doi: 10.1002/humu.20665. [DOI] [PubMed] [Google Scholar]
- 49.Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, et al. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- 50.Schmahl J, Yao HH, Pierucci-Alves F, Capel B. Colocalization of WT1 and cell proliferation reveals conserved mechanisms in temperature-dependent sex determination. Genesis. 2003;35:193–201. doi: 10.1002/gene.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144:3237–3243. doi: 10.1210/en.2002-0095. [DOI] [PubMed] [Google Scholar]
- •52.Smith CA, Shoemaker CM, Roeszler KN, Queen J, Crews DP, Sinclair AH. Cloning and expression of R-Spondin1 in different vertebrates suggests a conserved role in ovarian development. BMC Dev Biol. 2008;8:72. doi: 10.1186/1471-213X-8-72. This study argues for a conserved role of Rspondin-1 in vertebrate sex determination, given its close association with the female pathway in mice, chickens, and turtles.
- 53.Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- 54.Ross AJ, Tilman C, Yao H, MacLaughlin D, Capel B. AMH induces mesonephric cell migration in XX gonads. Mol Cell Endocrinol. 2003;211:1–7. doi: 10.1016/j.mce.2003.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pieau C, Dorizzi M. Oestrogens and temperature-dependent sex determination in reptiles: all is in the gonads. J Endocrinol. 2004;181:367–377. doi: 10.1677/joe.0.1810367. [DOI] [PubMed] [Google Scholar]
- 56.D’Cotta H, Fostier A, Guiguen Y, Govoroun M, Baroiller JF. Aromatase plays a key role during normal and temperature-induced sex differentiation of tilapia Oreochromis niloticus. Mol Reprod Dev. 2001;59:265–276. doi: 10.1002/mrd.1031. [DOI] [PubMed] [Google Scholar]


