Figure 8.
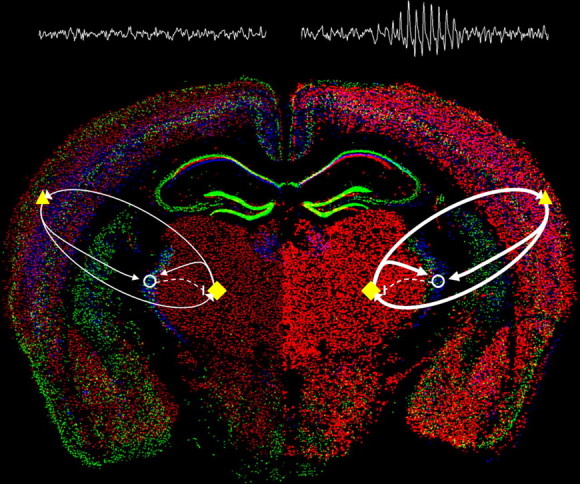
Enhanced α1G-mediated thalamocortical network activity evokes absence seizures. Model of relative changes in thalamocortical activity arising from selective enhancement of α1G T-type calcium channel expression within the brain (WT, left; α1G transgenic, right) illustrated by merged in situ hybridization images (courtesy of the Allen Brain Atlas) of the three T-type channel subunits (Cacna1g, red; Cacna1h, green; and Cacna1i, blue). Major elements of the critical thalamocortical loop consist of ascending TC cells (yellow diamonds) that excite (solid arrows) cortical cells (yellow triangles) and interneurons in the nRT (open circles), descending cortical cells that excite the TC and nRT, and nRT cells that inhibit (dashed line) the TC. Within the WT brain, a balanced network produces normal EEG activity, while in the α1G transgenic brain, transgenic overexpression of the Cacna1g gene (intense red cells) initiates hypersynchronized excitatory activity in the loop between the TC and cortex (bolder arrows), sufficient to generate SWDs and an absence epilepsy phenotype.
