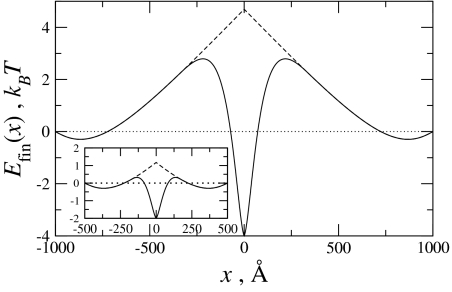
Fig. 6.
The interaction energy between finite molecules of length L = 1,000 Å (Inset, L = 500 Å). Differences between nonhomologous (dashed line) and homologous (solid line) molecules appear when the shift between the pairs is L/3 from full juxtaposition (see text). Homology reduces the energetic barrier for the sliding finite molecules, and for the electrostatic coefficients used here [the same as those used in Fig. 4, with a0 = 0.75·10−2 kBT/Å (16)], full juxtaposition of the homologous fragments is energetically favored.