Figure 6.
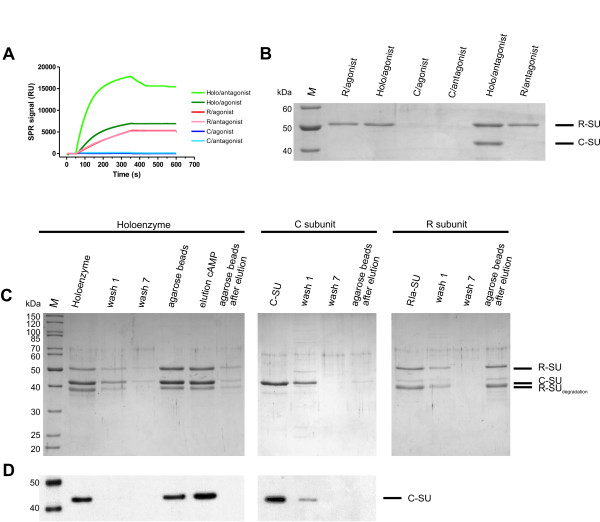
Rp-cAMPS analogs bind the intact PKA holoenzyme complex. The entire PKA type I holoenzyme complex (R2C2) binds highly specific to Rp-8-AHDAA-cAMPS as demonstrated by SPR (A), BIA MS (B) as well as affinity purification followed by SDS-PAGE (C) and Western blot analysis (D). (A) SPR binding pattern of type I holoenzyme, hRIα and Cα (as indicated, 250 nM each) on antagonist (Rp-8-AHDAA-cAMPS) and agonist (8-AHA-cAMP) surfaces. Measurements were performed in buffer A containing 0.005% P20, 1 mM ATP and 10 mM MgCl2 at a flow rate of 5 μL/min. (B) BIA-MS: SDS-PAGE analysis of protein recovered from the experiments depicted in (A). Type I holoenzyme, R-subunit and C-subunit were injected to the agonist and antagonist surfaces as described and were eluted with 0.2% SDS after a 4 min wash step. The eluted material from 15 repetitive runs was pooled and analysed by SDS-PAGE, MS and Western blot analysis (not shown). C-subunit (42 kDa) and R-subunit hRIα (50 kDa) are indicated. M: molecular weight marker (Page-Ruler, unstained protein ladder, Fermentas). (C) Binding of PKA type I holoenzyme complex (R2C2, left panel), free C-subunit (center panel) and free R-subunit (right panel) to antagonist agarose (Rp-8-AHA-cAMPS, 600 pmoles, for chemical structure see Fig. 1C) determined with SDS-PAGE. (D) Immunoblot analysis of SDS gels from panel (C) using anti-C-subunit antibody.