Abstract
Early ontogenetic adaptations reflect the evolutionary history of a species. To understand the evolution of the deep-sea fauna and its adaptation to high pressure, it is important to know the effects of pressure on their shallow-water relatives. In this study we analyse the temperature and pressure tolerances of early life-history stages of the shallow-water species Mytilus edulis. This species expresses a close phylogenetic relationship with hydrothermal-vent mussels of the subfamily Bathymodiolinae. Tolerances to pressure and temperature are defined in terms of fertilization success and embryo developmental rates in laboratory-based experiments. In M. edulis, successful fertilization under pressure is possible up to 500 atm (50.66 MPa), at 10, 15 and 20°C. A slower embryonic development is observed with decreasing temperature and with increasing pressure; principally, pressure narrows the physiological tolerance window in different ontogenetic stages of M. edulis, and slows down metabolism. This study provides important clues on possible evolutionary pathways of hydrothermal vent and cold-seep bivalve species and their shallow-water relatives. Evolution and speciation patterns of species derive mostly from their ability to adapt to variable environmental conditions, within environmental constraints, which promote morphological and genetic variability, often differently for each life-history stage. The present results support the view that a direct colonization of deep-water hydrothermal vent environments by a cold eurythermal shallow-water ancestor is indeed a possible scenario for the Mytilinae, challenging previous hypothesis of a wood/bone to seep/vent colonization pathway.
Keywords: pressure, temperature, Mytilidae, evolution, shallow water, deep sea
1. Introduction
Bacterial and higher animal life has been found in the deepest parts of the ocean, including the Challenger Deep in the Marianas Trough, where pressure is near 1100 atm. Hypotheses on the origins of the deep-sea fauna say that extant deep-sea organisms dispersed for long distances through isothermal water masses in the past glacial periods (Tyler et al. 2000; Thatje et al. 2005). Therefore, today's faunal exchange between deep-sea and shallow-water animals could occur via cold-adapted species living at high latitudes, where the water column is isothermal (Kussakin 1973; Menzies et al. 1973; Hessler & Thistle 1975; Hessler & Wilson 1983; Tyler & Young 1998; Thatje et al. 2005). At lower latitudes, during the Mesozoic and early Cenozoic, the deep sea was warmer than at present, and could have allowed shallow-water species to invade deeper ocean habitats (Menzies et al. 1973; Benson 1975; Berger 1979; Schopf 1980; Hessler & Wilson 1983; Young et al. 1997). To further elucidate this hypothesis, increasing relevance has recently been given to studies on pressure and thermal tolerance of the early life stages of both shallow-water and deep-sea invertebrates, together with studies on past changes in deep-sea hydrography (Tyler et al. 2000; Tokuda et al. 2006; Pradillon & Gaill 2007).
In the marine environment, pressure is the single variable that has a continuous relationship with depth, increasing by approximately 1 atm (atmosphere; 105 Pascals) per 10 m of water depth. Marine species' habitats are often also defined in relation to upper and lower depth limits and these limits are ultimately related to pressure tolerances of the organisms. Ideally, these habitat boundaries should be assessed for each life stage, as the depth tolerance range in each stage is different in some species (Anger 2001; Aquino-Souza et al. 2008). The early life stages of marine benthic invertebrates, for instance, are the most likely opportunities they have to disperse over long distances and to colonize new habitats (Tyler 1995; Young et al. 1996; Macdonald 1997; Tyler & Young 1998).
An ecological factor of great importance in defining the distribution of species is the temperature. The temperature tolerances for each life stage of a marine invertebrate can vary in a single species and affect the survival and the ability of a species to colonize new habitats. Temperature accelerates or delays the rates of metabolism and thus affects larval growth, development and survival as well as seasonal variation in the occurrence of larvae in the plankton (Anger 2001; for review see Clarke 2003). A stenothermal response is generally associated with species from the tropics or high latitudes, while those adapted to pronounced seasonal and regional temperature variations are typical of intermediate climatic zones and show a eurythermal response (Anger 2001, and references therein).
In order to understand the evolution/adaptation of the deep-sea fauna in terms of its sensitivity to pressure, it is important to know how their biological structures and processes differ from their shallow-water relatives (Childress & Fisher 1992; Somero 1992). These questions motivated scientists to develop high-pressure equipment to study the effect of pressure on marine organisms (Quetin & Childress 1980; Young & Tyler 1993; Young et al. 1996; Shillito et al. 2001). Furthermore, pressure is a physical property affecting molecular interactions and in consequence all biological processes on the Earth, and is largely unaffected by other factors. According to Le Chatelier's principle, if a chemical system at equilibrium experiences a change in concentration, temperature, volume or total pressure, the equilibrium will shift in order to counteract the imposed change (Somero 1992; Pradillon & Gaill 2007).
In biological systems the effect of pressure causes a compression of the system, conditioning physiological and biochemical processes involved in increasing or decreasing cell volume. A combination of the pressure effect with other physico-chemical factors affecting biological processes, e.g. temperature, pH and salinity, will intensify or reduce the effect of pressure alone. Pressure sensitivities of enzymes, structural proteins and membrane-based systems differ markedly between shallow-water and deep-sea species (Somero 1992; Pradillon & Gaill 2007).
The subfamily Mytilinae is the shallow-water closest taxonomic group to the hydrothermal vent and seep mussels of the subfamily Bathymodiolinae (Distel et al. 2000). Jones et al. (2006) revealed that vent species evolved multiple times, with moments of habitat reversals, but evidence is that there was a progressive evolution from shallow to deep habitats. Mid-ocean hydrothermal vent species may represent a monophyletic group with one noticeable reversal, and this is in agreement with previous hypotheses regarding evolution from wood/bone to seeps/vents (Jones et al. 2006).
The blue mussel Mytilus edulis Linné, 1758, is a widespread semi-sessile epibenthic bivalve found on rocky shores, shallow sublittoral zones and estuaries (Newell 1989). It is very common in northern Europe and parts of the Atlantic coast off Canada, but also colonizes temperate zones in the Southern Hemisphere (Gosling 1992). The upper vertical limit of M. edulis populations' distribution on rocky shores is determined by its tolerance to temperature and desiccation (Seed & Suchanek 1992). This species is relatively tolerant to extreme cold and freezing and it can survive occasional short frost events, but may not be resistant to persistent very low temperatures (Bourget 1983). The lower depth limit of distribution of M. edulis is presumably mainly influenced by predation (Seed 1969). In addition, the burial and abrasion by shifting sands are also of relevant importance (Daly & Mathieson 1977; Holt et al. 1998). The maximum depth distribution registered for this species in the Baltic Sea is 40 m, and its depth limit distribution is associated with the presence/absence of a hard bottom substratum (Bubinas & Vaitonis 2003). Subtidal populations have been reported on seamounts, dock pilings and offshore oil platforms, where they grow to a larger size, probably owing to a lack of predators (Seed & Suchanek 1992). Uncommonly, it has been found in deeper and cooler waters (100–499 m, Theroux & Wigley 1983) owing to shallower lack of habitat and/or presence of hard substratum at greater depths. The genus Mytilus is also an important invasive species (Carlton 1999).
Mytilus edulis is dioecious with rare occasions of hermaphroditism (Seed 1976; Micallef & Tyler 1988), and has a large number of small-sized eggs and a planktotrophic larva (Bayne 1976). Gametogenesis is synchronous and spawning occurs when eggs and sperm are fully ripe and are released through the exhalent siphon into the water column where fertilization takes place (Bayne 1976; Newell 1989). The embryonic development takes a few days and comprises cleavages, the formation of cilia, velum and shell gland—trochophore stage, up to the formation of the D-shaped shelled larvae, when it starts its planktotrophic phase (Bayne 1976). The depth distribution of larvae and juveniles of M. edulis is presumed to be the same as in adults (Newell 1989).
In the present study, the embryonic and larval development of M. edulis, along its entire physiological temperature and pressure tolerance window, is analysed, to our knowledge, for the first time. The physiological tolerances of the early life stages are examined, as they are of extreme importance in controlling the distribution, colonization pathways and to some extent the speciation within closely related species. The results are discussed in the frame of current theories on the evolution of the macrofauna in chemosynthetic environments.
2. Material and methods
(a) Sampling and spawning
Adult specimens of M. edulis were collected from Southampton Water (UK) and maintained at 15±1.5°C in a running seawater system in the aquarium of the National Oceanography Centre, Southampton, where the experimental work took place in June 2007. Spawning was induced by heavily shaking all mussels in a bucket (mechanical shock; Costello et al. 1957; Sprung & Bayne 1984), followed by the injection of 1 ml of 0.55 M KCl into the mantle cavity of each mussel (salinity shock; Young & Tyler 1993). To obtain gametes, each mussel was placed in individual glass bowls and left ‘dry’ for 10 min, with an ambient temperature of approximately 20°C. The mussels were submerged with 5°C (temperature shock; Costello et al. 1957; Sprung & Bayne 1984) filtered seawater (1.6 μm retention) and spawning started after temperature inside the bowls exceeded 15°C.
(b) Temperature effect on embryonic and larval development
Three cultures of fertilized eggs were prepared by mixing freshly spawned male and female gametes in a 1 l beaker in filtered seawater (1.6 μm retention filter) at 15°C. Gametes from each male/female pair were used in each culture. Fertilized eggs were transferred into 20 ml glass vials and incubated at 5, 10, 15, 20 and 25°C, at atmospheric pressure. Cultures were sampled regularly (every 10 min in the first 4 hours after fertilization, and once per hour thereafter) and the time from fertilization and stage of development were noted, until the D-larvae stage was reached for all temperatures. The chosen stages of development were defined according to distinguishable morphological features between stages (table 1), observable under a compound microscope.
Table 1.
Classification of early ontogenetic stages in M. edulis, with reference to the main characteristics observed in each stage (changed after Zardus & Martel 2002).
| stage | main characteristics |
|---|---|
| A | abnormal development |
| 0 | unfertilized egg; no sign of polar body |
| I | fertilized, uncleaved egg; showing polar body (and polar lobe formation) |
| II | two-cell stage; first cleavage and extrusion of polar body |
| III | four-cell stage; one large D-cell and three smaller cells |
| IV | eight-cell stage; showing spiral unequal cleavage of blastomeres |
| V | multi-cell; unequal cleavage producing micromeres in the animal pole and macromeres in the vegetal pole; within the fertilization membrane |
| VI | early blastula; released from the fertilization membrane; developing cilia |
| VII | gastrula; with blastopore (developing gut); invagination of the shell field (developing shell); quadrants with cilia |
| VIII | early trochophore; developing apical sense organ (apical plate+apical tuft) |
| IX | late trochophore; with velum and organic pellicule of first shell |
| X | D-larva; free-swimming straight hinge veliger with fully formed velum and prodissoconch I |
(c) Pressure effect on embryonic and larval development with fertilization under pressure
The embryonic developmental stage, after 4 and 24 hour treatments, was assessed for pressures of 1, 100, 200 and 300 atm (plus 400 and 500 atm for 4 hour treatments only, as the 24 hour treatment performed first indicated that successful fertilization was likely at greater pressures) and at temperatures of 10, 15 and 20°C, with fertilization occurring under pressure. Gametes from three different males and three different females were used, so that three replicates (each from one male/female pair) were assigned to each pressure/temperature combination.
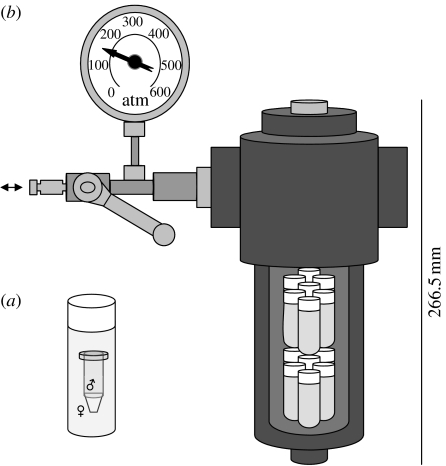
The following method was designed to prevent fertilization before pressurising eggs and sperm, and at the same time allowing them to mix under pressure: eggs from each female were collected from the glass bowls and re-suspended in ambient seawater, in a 1 l beaker, and then transferred into 6 ml plastic vials; 0.5 ml of diluted sperm suspension was pipetted into a 1 ml microcentrifuge tube (leaving 0.5 ml of air space); one microcentrifuge tube containing the sperm suspension was inserted into each plastic vial containing the egg suspension (figure 1a); the plastic vial was refilled with the egg suspension until it overflowed and the cap closed, avoiding any air being trapped inside; plastic vials were placed inside the pressure vessel (figure 1b) and filled with tap water at the test temperature; the pressurization to the desired pressure was continuous. When pressurising the vessels, it was possible to hear the microcentrifuge tubes imploding inside the plastic vials at approximately 50 atm, due to air left inside on purpose. In all treatments, pressurization was continuous and took at most 10 s until reaching the desired experimental pressure level.
Figure 1.
Schematic of the experimental pressure vessels: (a) plastic vial filled with the egg suspension and the microcentrifuge tube half-filled with sperm suspension; (b) pressure vessel showing the plastic vials inside (arrow indicates the connection valve to the hydraulic pump).
For the atmospheric pressure cultures, fertilization occurred when eggs and sperm were mixed in a 1 l beaker and the solution was transferred into 6 ml plastic vials. Pressure chambers and 1 atm cultures were incubated at 10, 15 and 20°C for 4 and 24 hours. At the end of each trial, pressure vessels were depressurized and samples for the analysis of the developmental stage were quickly preserved in 4 per cent formalin. Fifty embryos from each replicate were randomly selected and staged according to the embryonic development.
After depressurization, sub-samples from the vials containing embryos were immediately preserved in 3 per cent glutaraldehyde in 0.1 M phosphate buffer, pH 7.2, and kept refrigerated at 4°C until used for scanning electron microscopy (SEM). Samples were subsequently rinsed with buffer, dehydrated and critical point dried. Specimens were then mounted on stubs and sputter-coated in gold. SEMs were taken using a Hitachi S800 SEM.
(d) Pressure effect on embryonic and larval development with fertilization at atmospheric pressure
For the treatments with fertilization occurring at atmospheric pressure, analysing at 50 hours of embryonic and larval development, three cultures of fertilized eggs were prepared by mixing freshly spawned male and female gametes in a 1 l beaker in filtered seawater (1.6 μm retention) at 15°C (ambient temperature). Gametes from each male/female pair were used in each culture. Fertilized eggs were transferred into 6 ml plastic vials, filled until overflowing and with the cap closed to avoid any air being trapped inside. Plastic vials were placed inside the pressure chambers, filled with tap water at the test temperature, and the pressurization to the desired pressure was continuous. The fertilized eggs were incubated at 1, 100, 200 and 300 atm, and 5, 10, 15, 20 and 25°C. After 50 hours, pressure vessels were depressurized and all samples were quickly preserved in 4 per cent formalin. Fifty embryos from each replicate were randomly selected and staged according to the embryonic development.
(e) Statistical analyses
Data on the proportion of abnormally developing embryos present in each culture failed the assumption of normality, even after the data were arcsine transformed. The raw data were used in the non-parametric Kruskal–Wallis single factor analysis of variance by ranks to test for temperature effects and pressure effects on the proportion of abnormally developing embryos. Temperature and pressure effects were tested separately, for each treatment and each incubation period (e.g. results of pressure effects are for each temperature tested in each incubation period).
3. Results
(a) Temperature effects on embryonic and larval development
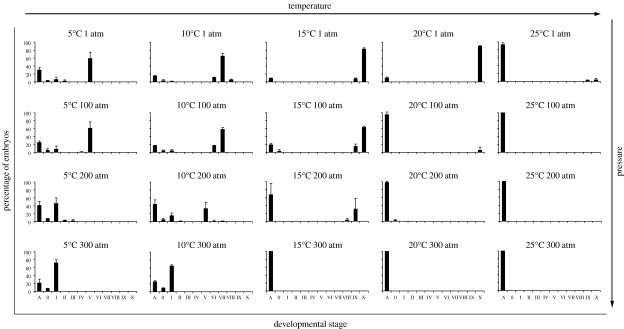
Mytilus edulis embryos develop faster at higher temperatures. For example, at 1 atm most embryos incubated at 5°C require 50 hours to reach stage V (multi-cell; figure 2; table 1) but reached stage X (D-larva) at 20°C. At 10°C and after 4 hours of incubation, the embryos developed to stage III (four-cell stage; figure 3), after 24 hours they reached stage VI (early blastula; figure 4) and past 50 hours they reached stage VIII (early trochophore). After 4 hours incubation at 15 and 20°C, the embryos developed to stage V, past 24 hours, the embryos reared at 15°C were in stage VII (gastrula) and those reared at 20°C reached stage X. The embryos incubated at 15°C needed 50 hours to get to D-larvae stage. At 25°C, those that survived 50 hours incubation reached stages IX and X (late trochophore and D-larvae, respectively), although 93 per cent of the embryos were developing abnormally or dying, and the culture was quickly degrading.
Figure 2.
Mytilus edulis embryonic and early larval development incubated at different pressure/temperature regimes for 50 hours, with fertilization occurring at atmospheric pressure. Histograms are of percentage mean and standard deviation. Stages of development: A=abnormal; 0=unfertilized; I=fertilized, uncleaved egg; II=two-cell; III=four-cell; IV=eight-cell; V=multi-cell; VI=early blastula; VII=gastrula; VIII=early trochophore; IX=late trochophore; X=D-larva (see table 1 for a detailed description of each developmental stage).
Figure 3.
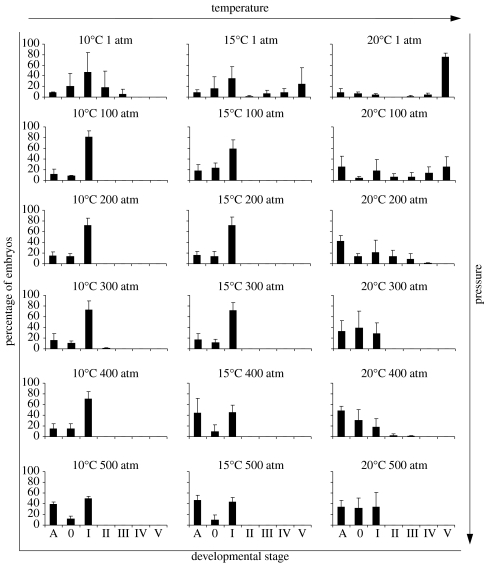
Mytilus edulis embryonic development incubated at different pressure/temperature regimes for 4 hours, with fertilization occurring under pressure. Histograms are of percentage mean and standard deviation. Stages of development: A=abnormal; 0=unfertilized; I=fertilized, uncleaved egg; II=two-cell; III=four-cell; IV=eight-cell; V=multi-cell (see table 1 for a detailed description of each developmental stage).
Figure 4.
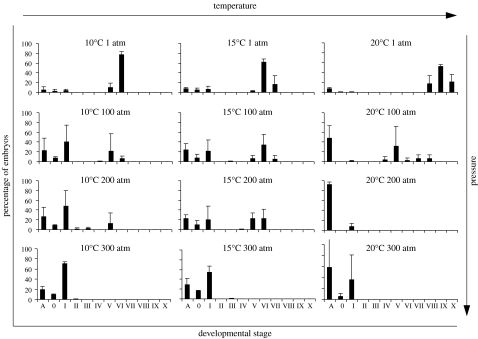
Mytilus edulis embryonic and early larval development incubated at different pressure/temperature regimes for 24 hours, with fertilization occurring under pressure. Histograms are of percentage mean and standard deviation. Stages of development: A=abnormal; 0=unfertilized; I=fertilized, uncleaved egg; II=two-cell; III=four-cell; IV=eight-cell; V=multi-cell; VI=early blastula; VII=gastrula; VIII=early trochophore; IX=late trochophore; X=D-larva (see table 1 for a detailed description of each developmental stage).
When testing for effects of temperature in the proportion of abnormally developing embryos, reared at atmospheric pressure, it is possible to detect a significant effect in the 50 hours incubation period when all temperatures are analysed together (5–25°C; Kruskal–Wallis H=6.058, p=0.011; table 2). There is no significant effect of temperature (tested temperatures: 10, 15 and 20°C) in the proportion of embryos developing abnormally for incubation periods of 4 hours and 24 hours for any pressure treatment (table 2).
Table 2.
Kruskal–Wallis analysis of variance testing the effects of temperature (10, 15 and 20°C) on the proportion of abnormally developing embryos reared at the different pressures for different incubation periods. (H statistic (degrees of freedom, N=number of replicates); *for temperatures: 5, 10, 15, 20 and 25°C.)
| incubation period | ||||||||
|---|---|---|---|---|---|---|---|---|
| 4 hours | 24 hours | 50 hours | 50 hours* | |||||
| pressure (atm) | H (2, N=9) | p-value | H (2, N=9) | p-value | H (2, N=9) | p-value | H (4, N=15) | p-value |
| 1 | 0.073 | 0.964 | 0.615 | 0.735 | 6.058 | 0.048 | 13.099 | 0.011 |
| 100 | 0.610 | 0.737 | 2.443 | 0.295 | 5.695 | 0.058 | 12.603 | 0.013 |
| 200 | 5.728 | 0.057 | 5.600 | 0.061 | 4.545 | 0.103 | 11.070 | 0.026 |
| 300 | 1.689 | 0.430 | 1.107 | 0.575 | 7.624 | 0.022 | 13.086 | 0.011 |
(b) Pressure effects on embryonic and larval development
Fertilization under pressure succeeded in all pressure treatments (figures 3 and 4). After 4 hours incubation, in both 10 and 15°C treatments for 100–500 atm, eggs were fertilized but did not develop (figure 3). A small proportion of embryos reached stage V (multi-cell), when incubated for 4 hours at 20°C per 100 atm, but from 200 atm up to 500 atm embryos were in less advanced stages, indicating a retarded development. For the 20°C cultures, with increasing pressure the number of abnormal individuals increased (figure 3).
In the embryos exposed for 24 hours, the maximum developmental stage reached was for the cultures reared at atmospheric pressure (figure 4). A similar pattern occurred for the cultures at 100 atm with a maximum development for the 20°C treatment: stage VIII (early trochophore). At 20°C per 200 atm more than 92 per cent of the embryos did undergo abnormal development, while at 15°C per 200 atm embryos reached stage VI, and at 10°C per 200 atm a small portion of embryos developed to stage V (figure 4). At 300 atm and for all temperatures tested, embryos did not develop. In all temperatures, an increase of abnormal development with increasing pressure was observed.
The cultures exposed to 100 atm for 50 hours and incubated at 5, 10 and 15°C behaved very similarly to those reared at atmospheric pressure, while at higher temperatures most of embryos were abnormal (figure 2). In both 20 and 25°C cultures exposed to 200 and 300 atm, the embryos were abnormal or died. In the cultures reared at 300 atm at 5 and 10°C, embryos did not develop, and most of them remained in stage I—uncleaved showing a polar body—after fertilization (figure 2). On the other hand, at 10°C per 200 atm more than 30 per cent of embryos reached stage V—multi-cell stage (figure 2). At 15°C per 200 atm, the few embryos developing normally reached stage IX (figure 2). In general, the degree of abnormal development observed in embryos increases with both increased pressure and temperature.
Abnormal cleavages, the lack of cell membrane, and extrusions of the cytoplasm originating knob-like structures on the exterior of the embryo are the common abnormalities observed in pressurized cultures (see figures in the electronic supplementary material). The effect of pressure on the proportion of abnormally developing embryos was tested for each temperature and each incubation period (table 3). Results show no significant effect of pressure on all the temperatures analysed (10, 15 and 20°C) for both the incubation periods of 4 and 24 hours, except for treatment of 20°C per 24 hours (Kruskal–Wallis H=8.128, p=0.043; table 3). With a longer incubation period of 50 hours, the effect of pressure on the proportion of abnormally developing embryos is significant for all temperatures tested (table 3), except for the 5°C cultures (Kruskal–Wallis H=6.407, p=0.093; table 3).
Table 3.
Kruskal–Wallis analysis of variance testing the effects of pressure (1, 100, 200 and 300 atm) on the proportion of abnormally developing embryos reared at different temperatures for different incubation periods. (H statistic (degrees of freedom, N=number of replicates).)
| incubation period | ||||||
|---|---|---|---|---|---|---|
| 4 hours | 24 hours | 50 hours | ||||
| temperature (°C) | H (3, N=12) | p-value | H (3, N=12) | p-value | H (3, N=12) | p-value |
| 5 | — | — | — | — | 6.407 | 0.093 |
| 10 | 1.568 | 0.667 | 4.351 | 0.226 | 10.157 | 0.017 |
| 15 | 1.977 | 0.577 | 6.590 | 0.086 | 10.237 | 0.017 |
| 20 | 5.100 | 0.165 | 8.128 | 0.043 | 8.194 | 0.042 |
| 25 | — | — | — | — | 10.735 | 0.013 |
4. Discussion
(a) Methodological considerations
The method to induce spawning used in this work results from a combination of different approaches suggested in the literature (e.g. Costello et al. 1957; Sprung & Bayne 1984; Young & Tyler 1993). The mechanical, temperature and salinity shocks proved to be efficient, with at least 15 per cent of mussels spawning per trial.
Fertilization under pressure was 100 per cent effective as all the microcentrifuge tubes imploded under pressure allowing fertilization to take place. This new and simple technique may be used in future pressure studies as a way to mix small volumes of solutions under pressure. As fertilization rates at atmospheric pressure and at tolerated pressures (i.e. 100 atm at 10 and 15°C, figures 2–4) are comparable, we do not assume imploding microtube vials to significantly bias the fertilization process, i.e. by lysing cells. The ‘popping microcentrifuge’ tube approach of fertilization has the advantage of not affecting the pH or seawater density. When developing the experimental design, dissolvable capsules yielded little satisfying results as the dissolving capsules drastically affected the viscosity of small volumes of seawater. Fertilization of eggs following rupture of microcentrifuge tubes at approximately 50 atm and prior to reaching the desired experimental pressure is unlikely. Pressurization was continuous and only lasted 5–7 s following rupture of microcentrifuge tubes. Successful fertilization in M. edulis eggs took between 40 and approximately 60 s when studied at 1 atm under a compound microscope and within the temperature range tested.
The present work focuses on the study of the early embryonic phase of the M. edulis life cycle that corresponds to the full embryogenic period, from fertilization to the early D-larvae stage, when embryos do not feed and rely on nutritional reserves of maternal origin present in the oocytes. In our study, gametogenesis in M. edulis took place at approximately 15°C, the maintenance temperature of adult blue mussels. Pre-spawning temperatures may affect physiological tolerances in the offspring and thus future study should focus on the effect of temperature on offspring quality in M. edulis. Whereas survival rates in embryos and larvae might be enhanced if acclimation to the experimental temperature would include gametogenesis, we do not believe that the overall physiological tolerance pattern found in this study would have changed.
(b) Temperature and pressure effect on embryogenesis
Our data suggest that at atmospheric pressure the temperature tolerance window for successful early embryogenesis of M. edulis ranges from approximately 10–20°C. The embryo development scales with temperature (for review see Anger 2001), i.e. embryos take more time at lower temperatures to reach the same embryonic stage than those reared at higher temperatures. At higher temperatures, close to thermal limits, a higher proportion of abnormally developing embryos are observed. This is probably related to the metabolic rates increasing with increasing temperatures; although temperatures might not yet have reached a lethal state, more energy has to be allocated to the metabolism, which negatively affects the embryo development.
Successful embryo development is possible from 1 atm up to 500 atm, which was the maximum pressure condition tested. Active sperm cells were observed under a compound microscope straight after depressurization when exposed at 500 atm for 4 hours. With the presented pressure range tested, we hypothesize that pressure presents no barrier to fertilization. It is possible to observe a slower development with increasing pressure, as well as an increase of abnormal development of the embryos. Standard deviations potentially reflect the intraspecific variability found in the early ontogeny of M. edulis (figure 3), which is higher in the amount of unfertilized eggs and abnormal embryos. Most of the abnormalities observed are due to rupture of the egg membrane, while cleavages apparently continue occurring. The egg fertilization membrane is only supposed to break after the blastula stage (Zardus & Martel 2002), but was observed for earlier stages of development for pressures ≥200 atm (figure S2c in the electronic supplementary material).
The cytoplasmic extrusions observed in pressurized embryos (figures S1b,d and S2c in the electronic supplementary material) have been described in pressurized protozoans (Landau et al. 1954; Kitching 1957). According to these authors, elevated pressures cause (i) a reduction in the protoplasmic gel strength, and (ii) a weakness of the more rigid cortical portions of the cell. These effects apparently lead to a separation of the cell membrane from the cytoplasm, and to the formation of knob-like structures in the exterior of the cell membrane—ultimately leading to cell-membrane breakage. Zimmerman & Marsland (1964) found that high pressure affects the mitotic apparatus of dividing cells. In the cleaving eggs of the sea urchin Arbacia punctulata, chromosomal movements become retarded at 140 atm and are completely arrested at 280 atm. This effect is in general reversible, but when subject to a pressure in excess of 544 atm for more than 5 min leads to a drastic and irreversible disorganization of the mitotic apparatus (Zimmerman & Marsland 1964). These experiments were for periods of 1–15 min with fertilization at atmospheric pressure. The authors argue that recovery might be possible, but at the same time the disorganization of the mitotic apparatus observed after a maximum pressurization time of 15 min could be permanently compromised.
The embryonic development in some shallow-water marine invertebrates is inhibited when subjected to high pressures (Marsland 1950; Salmon 1975), and this may limit the vertical distributions of species (Young & Tyler 1993). Pressure can even act differently for each early life-history stage, as found in the sea-urchin Psammechinus miliaris, in which the pelagic larva is more resilient to high pressure than the earlier embryonic stages (Aquino-Souza et al. 2008). With the results presented here, it would be possible to estimate that the theoretical maximum distribution depth for the M. edulis embryos is, at least 2000 m for the temperatures tested. Marsland (1950, 1970) found that in dividing marine eggs, the higher the temperatures, within the temperature tolerance range of a species, the stronger the cortical gel structures and their resistance to high pressures. Although, our results are contrary to this observation, as for example in the 50 hours treatment when comparing the cultures pressurized at 100 atm at 15 and 20°C, at 15°C embryos are more tolerant to pressure and develop to D-larvae, with no increment on the proportion of abnormally developing embryos.
Metabolic rates increase with increasing temperature (for review see Clarke 2003). In the case of M. edulis, the lower the pressure the faster the embryonic development. In the present study, a prolonged time of exposure to the different temperature/pressure combinations gives us a further view on this developmental process: after 4 hours of exposure, at 20°C we see higher tolerance to high pressure, as cleavages occur and the number of abnormalities is low. After 24 hours of exposure, it is possible to observe that those embryos undergo irregular cleavages and this may indicate that further successful progression in development is unlikely under high pressure. After 50 hours this is a fact, as embryos do not tolerate pressures greater than or equal to 100 atm.
Our results show embryos developing normally at high pressures, and low temperatures. Questions remain about whether embryos developing normally under pressure, but with a slower growth rate due to low temperature, would become abnormal as the complexity of developing embryos increases. We assume that there are particular stages in the early ontogeny which are crucial to tolerate pressure. When an embryo reaches these stages it will be defined whether there will be normal development or not. In similar pressure/temperature studies, evidence suggests that low temperature retards the embryonic development (e.g. Young et al. 1997; Tyler & Young 1998), and in the case of M. edulis pressure combined with temperature favours lower temperatures where fewer abnormalities are observed. Thus it is reasonable to hypothesize that the invasion of the deep sea by M. edulis is possible in terms of pressure tolerances in embryos and larvae.
(c) Seeding the deep sea
Wood-, bone-, vent- and seep-associated mussels are phylogenetically closely related and are indicative of a recent common ancestry for vent and non-vent species (Distel et al. 2000). A long isolation of this group of chemosynthetic mussels is proposed to be the cause of divergence from other mytilids from shallow water (Distel et al. 2000). It has been hypothesized that wood- and bone-associated mussels worked as an evolutionary step for shallow-water mussels to colonize hydrothermal vents and cold seeps (Distel et al. 2000). Deep-sea colonization by shallow-water species over evolutionary periods of time needs cold-stenothermy adapted species finding a rather stable, low temperature environment, which resulted in the concept of faunistic exchange through low temperature, isothermic water bodies (Kussakin 1973; Menzies et al. 1973; Hessler & Thistle 1975; Hessler & Wilson 1983; Thatje et al. 2005). Contrarily, the colonization of deep-water chemosynthetic environments would indeed require physiological properties in invertebrates that tolerate greater fluctuations in temperature (=cold-eurythermy), and the present study clearly demonstrated that blue mussels are a likely proof for such a scenario, which is furthermore supported by their close phylogenetic relationship with the Bathymodilinae (Distel et al. 2000) and similarities in their reproductive and larval developmental cycle (Lutz et al. 1980; Berg 1985; Kenk & Wilson 1985). Supporting this view of similar physiological prerequisites in both temperate shallow-water and hydrothermal vent species, bivalves from deep-sea hydrothermal vents have also shown growth rates similar to mytilids found in shallow-water environments and their growth rates are much higher (several orders of magnitude) than those in bivalve species living in non-chemosynthetic deep-sea habitat (Lutz et al. 1985). It might be challenging to suggest that from an evolutionary perspective, hydrothermal vent environments have been the only places where shallow-water temperate to sub-tropical invertebrates were able to establish, owing to similar temperature-tolerance regimes. Similar establishment in low-temperature deep-sea environments can only occur if the species possess a cold stenothermy to a constant, low-temperature environment—a physiological pattern, we hypothesize, not favoured in shallow-water species from sub-tropical to temperate regions. We propose that the development of a thermocline is not necessarily a physiological barrier to pelagic larval stages and drifting stages of any kind to penetrate the deep sea, although its physical properties may influence the direct settlement by invertebrate larvae into deeper waters.
The results presented here challenge the idea by Distel et al. (2000) that mytilids of the genus Bathymodiolus did colonize eurythermal vent sites via cold-stenothermal chemosynthetic wood and cold-seep environments. This evolutionary pathway would imply that Bathymodiolus regained cold eurythermy to colonize hydrothermal vents following the colonization of cold-stenothermal chemosynthetic environments—an evolutionary step that is generally assumed highly unlikely. The herein presented results indeed support the fact that for species of the Mytilinae, a direct colonization from shallow water into deep-water sites that both demand the same temperature tolerance is a more likely scenario and that the physiological basis of organismal biology should be increasingly taken into consideration when studying the evolutionary history of deep-sea faunas. It is possible that only advanced larvae have been involved in seeding deep-sea habitats over long evolutionary periods of time owing to lack of food at greater depth. Here, it must be reiterated, however, that invertebrate larvae can arrest development for substantial periods of time when encountering unsuitable habitat conditions such as lower temperatures in the colder water column, until encountering a more suitable habitat for development (Anger 2001; Thatje et al. 2004), which in addition should facilitate direct shallow water—deep water exchange of floating early life-history stages, provided a hyperbaric tolerance is expressed.
Acknowledgements
We would like to thank Dr Anton Page from the Biomedical Imaging Unit, School of Medicine, University of Southampton, for help in the preparation of samples for SEM. This research was funded by the European Commission through the MoMARNet Marie Curie Research Training Network of the FP6 (contract no. MRTN-CT-2003-505026), and was supported by the Marine Biodiversity and Ecosystem Functioning Network of Excellence MarBEF (contract no. GOCE-CT-2003-505446) of the FP6.
Supplementary Material
Examples of pressure effects on the morphology of M. edulis embryos incubated for 4 hours, with fertilization occurring at incubation pressure (light microscope images)
Examples of pressure effects on the morphology of M. edulis embryos incubated for 24 hours at 10°C, with fertilization occurring at incubation pressure
References
- Anger K. Crustacean Issues 14. A.A. Balkema Publishers; Rotterdam, The Netherlands: 2001. The biology of decapod crustacean larvae. [Google Scholar]
- Aquino-Souza R., Hawkins S.J., Tyler P.A. Early development and larval survival of Psammechinus miliaris under deep-sea temperature and pressure conditions. J. Mar. Biol. Assoc. UK. 2008;88:453–461. doi:10.1017/S0025315408001148 [Google Scholar]
- Bayne B.L. The biology of mussel larvae. In: Bayne B.L., editor. Marine mussels: their ecology and physiology. Cambridge University Press; New York, NY: 1976. pp. 81–120. [Google Scholar]
- Benson R.H. The origin of the psychrosphere as recorded in changes of deep-sea ostracod assemblages. Lethaia. 1975;8:69–83. doi:10.1111/j.1502-3931.1975.tb00919.x [Google Scholar]
- Berg C.J. Reproductive strategies of mollusks from abyssal hydrothermal vent communities. Bull. Biol. Soc. Wash. 1985;6:185–197. [Google Scholar]
- Berger W.H. Impact of deep-sea drilling on paleoceanography. In: Talwani M., Hay C.V., Ryan W.B.F., editors. Deep drilling results in the Atlantic Ocean: continental margins and paleoenvironment. American Geophysical Union; Washington, DC: 1979. pp. 297–314. [Google Scholar]
- Bourget E. Seasonal variations of cold tolerance in intertidal molluscs and their relation to environmental conditions in the St. Lawrence Estuary. Can. J. Zool. 1983;61:1193–1201. [Google Scholar]
- Bubinas A., Vaitonis G. The analysis of the structure, productivity, and distribution of zoobenthoscenoses in the Lithuanian economic zone of the Baltic Sea and the importance of some benthos species to fish diet. Acta Zool. Lituan. 2003;13:114–124. [Google Scholar]
- Carlton J.T. Molluscan invasions in marine and estuarine communities. Malacologia. 1999;41:439–454. [Google Scholar]
- Childress J.J., Fisher C.R. The biology of hydrothermal vent animals: physiology, biochemistry, and autotrophic symbioses. Oceanogr. Mar. Biol. Annu. Rev. 1992;30:337–441. [Google Scholar]
- Clarke A. Costs and consequences of evolutionary temperature adaptation. Trends Ecol. Evol. 2003;18:573–581. doi:10.1016/j.tree.2003.08.007 [Google Scholar]
- Costello D.P., Davidson M.E., Eggers A., Fox M.H., Henley C. Marine Biological Laboratory; Woods Hole, MA: 1957. Methods for obtaining and handling marine eggs and embryos. [Google Scholar]
- Daly M.A., Mathieson A.C. The effects of sand movement on intertidal seaweeds and selected invertebrates at Bound Rock, New Hampshire, USA. Mar. Biol. 1977;43:45–55. doi:10.1007/BF00392570 [Google Scholar]
- Distel D.L., Baco A.R., Chuang E., Morrill W., Cavanaugh C., Smith C.R. Marine ecology: do mussels take wooden steps to deep-sea vents? Nature. 2000;403:725–726. doi: 10.1038/35001667. doi:10.1038/35001667 [DOI] [PubMed] [Google Scholar]
- Gosling, E. M. 1992 Systematics and geographic distribution of Mytilus In The mussel Mytilus:ecology, physiology, genetics and culture. Developments in Aquaculture and Fisheries and Science (ed. E. M. Gosling), pp. 1–20. Amsterdam, UK: Elsevier.
- Hessler R.R., Thistle D. On the place of origin of deep-sea isopods. Mar. Biol. 1975;32:155–165. doi:10.1007/BF00388508 [Google Scholar]
- Hessler R.R., Wilson G.D.F. The origin and biogeography of malacostracan crustaceans in the deep sea. In: Sims R.W., Price J.H., Whalley P.E.S., editors. Evolution in time and space: the emergence of the biosphere. Academic Press; New York, NY: 1983. pp. 227–254. [Google Scholar]
- Holt T.J., Rees E.I., Hawkins S.J., Seed R. Biogenic reefs. An overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Volume IX. Scottish Association for Marine Science (UK Marine SACs Project); Peterborough, UK: 1998. [Google Scholar]
- Jones W.J., Won Y.-J., Maas P.A.Y., Smith P.J., Lutz R.A., Vrijenhoek R.C. Evolution of habitat use by deep-sea mussels. Mar. Biol. 2006;148:841–851. doi:10.1007/s00227-005-0115-1 [Google Scholar]
- Kenk V.C., Wilson B.R. A new mussel (Bivalvia, Mytilidae) from hydrothermal vents in the Galapagos Rift-Zone. Malacologia. 1985;26:253–271. [Google Scholar]
- Kitching J.A. Effects of high hydrostatic pressures on the activity of flagellates and ciliates. J. Exp. Biol. 1957;34:494–510. doi: 10.1242/jeb.51.2.319. [DOI] [PubMed] [Google Scholar]
- Kussakin O.G. Peculiarities of the geographical and vertical distribution of marine isopods and the problem of deep-sea fauna origin. Mar. Biol. 1973;23:19–34. doi:10.1007/BF00394108 [Google Scholar]
- Landau J.V., Zimmerman A.M., Marsland D.A. Temperature-pressure experiments on Amoeba proteus: plasmagel structure in relation to form and movement. J. Cell. Comp. Physiol. 1954;44:211–232. doi: 10.1002/jcp.1030440206. doi:10.1002/jcp.1030440206 [DOI] [PubMed] [Google Scholar]
- Lutz R.A., Jablonski D., Rhoads D.C., Turner R.D. Larval dispersal of a deep-sea hydrothermal vent bivalve from the Galapagos Rift. Mar. Biol. 1980;57:127–133. doi:10.1007/BF00387378 [Google Scholar]
- Lutz R.A., Fritz L.W., Rhoads D.C. Molluscan growth at deep-sea hydrothermal vents. Bull. Biol. Soc. Wash. 1985;6:199–210. [Google Scholar]
- Macdonald A.G. Hydrostatic pressure as an environmental factor in life processes. Comp. Biochem. Physiol. A. 1997;116:291–297. doi:10.1016/S0300-9629(96)00354-4 [Google Scholar]
- Marsland D.J. The mechanisms of cell division: temperature-pressure experiments on the cleaving eggs of Arbacia punctulata. J. Cell. Comp. Physiol. 1950;36:205–227. doi: 10.1002/jcp.1030360207. doi:10.1002/jcp.1030360207 [DOI] [PubMed] [Google Scholar]
- Marsland D. Pressure-temperature studies on the mechanisms of cell division. In: Zimmerman A.M., editor. High pressure effects on cellular processes. Academic Press; New York, NY: 1970. pp. 259–312. [Google Scholar]
- Menzies R.H., George R.Y., Rowe G.T. Wiley; New York, NY: 1973. Abyssal environment and ecology of the world oceans. [Google Scholar]
- Micallef S., Tyler P.A. Intrafollicular hermaphroditism in the marine mussel, Mytilus edulis L. Int. J. Invert. Reprod. Dev. 1988;14:47–52. [Google Scholar]
- Newell, R. I. E. 1989 Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (North and Mid-Atlantic)-blue mussel. U.S. Fish and Wildlife Service Biological Report 82 (11.102), U.S. Army Corps of Engineers, TR EL-82-4.
- Pradillon F., Gaill F. Pressure and life: some biological strategies. Rev. Environ. Sci. Biotech. 2007;6:181–195. doi:10.1007/s11157-006-9111-2 [Google Scholar]
- Quetin L.B., Childress J.J. Observations on the swimming activity of two bathypelagic mysid species maintained at high hydrostatic pressures. Deep-Sea Res. Part A. Oceanogr. Res. Pap. 1980;27:383–391. doi:10.1016/0198-0149(80)90033-3 [Google Scholar]
- Salmon E.D. Pressure-induced depolymerization of spindle microtubules. J. Cell Biol. 1975;65:603–614. doi: 10.1083/jcb.65.3.603. doi:10.1083/jcb.65.3.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf T.J.M. Harvard University Press; Cambridge, MA: 1980. Paleoceanography. [Google Scholar]
- Seed R. The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores. 2. Growth and mortality. Oecologia. 1969;3:317–350. doi: 10.1007/BF00390381. doi:10.1007/BF00390381 [DOI] [PubMed] [Google Scholar]
- Seed R. Ecology. In: Bayne B.L., editor. Marine mussels: their ecology and physiology. Cambridge University Press; New York, NY: 1976. pp. 13–65. [Google Scholar]
- Seed, R. & Suchanek, T. H. 1992 Population and community ecology of Mytilus In The mussel Mytilus:ecology, physiology, genetics and culture. Developments in Aquaculture and Fisheries and Science (ed. E. M. Gosling), pp. 87–169. Amsterdam, UK: Elsevier.
- Shillito B., Jollivet D., Sarradin P.-M., Rodier P., Lallier F.H., Desbruyères D., Gaill F. Temperature resistance of Hesiolyra bergi, a polychaetous annelid living on deep-sea vent smoker walls. Mar. Ecol. Prog. Ser. 2001;216:141–149. doi:10.3354/meps216141 [Google Scholar]
- Somero G.N. Adaptations to high hydrostatic pressure. Ann. Rev. Physiol. 1992;54:557–577. doi: 10.1146/annurev.ph.54.030192.003013. doi:10.1146/annurev.ph.54.030192.003013 [DOI] [PubMed] [Google Scholar]
- Sprung M., Bayne B.L. Some practical aspects of fertilizing the eggs of the mussel Mytilus edulis L. J. Conseil Internat. Expl. Mer. 1984;41:125–128. doi:10.1093/icesjms/41.2.125 [Google Scholar]
- Thatje S., Lovrich G.A., Anger K. Egg production, hatching rates, and abbreviated larval development of Campylonotus vagans Bate, 1888 (Crustacea: Decapoda: Caridea) in subantarctic waters. J. Exp. Mar. Biol. Ecol. 2004;301:15–27. doi:10.1016/j.jembe.2003.09.010 [Google Scholar]
- Thatje S., Hillenbrand C.D., Larter R. On the origin of Antarctic marine benthic community structure. Trends Ecol. Evol. 2005;20:534–540. doi: 10.1016/j.tree.2005.07.010. doi:10.1016/j.tree.2005.07.010 [DOI] [PubMed] [Google Scholar]
- Theroux, R. B. & Wigley, R. L. 1983 Distribution and abundances of east coast bivalve mollusks based on specimens in the National Marine Fisheries Service Woods Hole collections. NOAA Tech. Rep. NMFS SSRF-768, p. 172.
- Tokuda G., Yamada A., Nakano K., Arita N., Yamasaki H. Occurrence and recent long-distance dispersal of deep-sea hydrothermal vent shrimps. Biol. Lett. 2006;2:257–260. doi: 10.1098/rsbl.2005.0420. doi:10.1098/rsbl.2005.0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler P.A. Conditions for the existence of life at the deep-sea: an update. Oceanogr. Mar. Biol. Annu. Rev. 1995;33:221–244. [Google Scholar]
- Tyler P.A., Young C.M. Temperature and pressure tolerances in dispersal stages of the genus Echinus (Echinodermata: Echinoidea): prerequisites for deep-sea invasion and speciation. Deep-Sea Res. II. 1998;45:253–277. doi:10.1016/S0967-0645(97)00091-X [Google Scholar]
- Tyler P.A., Young C.M., Clarke A. Temperature and pressure tolerances of embryos and larvae of the Antarctic sea urchin Sterechinus neumayeri (Echinodermata: Echinoidea): potential for deep-sea invasion from high latitudes. Mar. Ecol. Prog. Ser. 2000;192:173–180. doi:10.3354/meps192173 [Google Scholar]
- Young C.M., Tyler P.A. Embryos of the deep-sea echinoid Echinus affinis require high-pressure for development. Limnol. Oceanogr. 1993;38:178–181. [Google Scholar]
- Young C.M., Tyler P.A., Gage J.D. Vertical distribution correlates with embryonic pressure tolerances in the deep-sea asteroid Plutonaster bifrons. J. Mar. Biol. Assoc. UK. 1996;76:749–757. [Google Scholar]
- Young C.M., Tyler P.A., Fenaux L. Potential for deep-sea invasion by Mediterranean shallow water echinoids: pressure and temperature as stage-specific dispersal barriers. Mar. Ecol. Prog. Ser. 1997;154:197–209. doi:10.3354/meps154197 [Google Scholar]
- Zardus J.D., Martel A.L. In: Atlas of marine invertebrate larvae. Young C.M., Sewell M.A., Rice M.E., editors. Academic Press; New York, NY: 2002. pp. 289–325. [Google Scholar]
- Zimmerman A.M., Marsland D. Cell division: effects of pressure on the mitotic mechanisms of marine eggs (Arbacia punctulata) Exp. Cell Res. 1964;35:293–302. doi: 10.1016/0014-4827(64)90096-5. doi:10.1016/0014-4827(64)90096-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examples of pressure effects on the morphology of M. edulis embryos incubated for 4 hours, with fertilization occurring at incubation pressure (light microscope images)
Examples of pressure effects on the morphology of M. edulis embryos incubated for 24 hours at 10°C, with fertilization occurring at incubation pressure