Abstract
For a dentition representing the most basal extant gnathostomes, that of the shark can provide us with key insights into the evolution of vertebrate dentitions. To detail the pattern of odontogenesis, we have profiled the expression of sonic hedgehog, a key regulator of tooth induction. We find in the catshark (Scyliorhinus canicula) that intense shh expression first occurs in a bilaterally symmetrical pattern restricted to broad regions in each half of the dentition in the embryo jaw. As in the mouse, there follows a changing temporal pattern of shh spatial restriction corresponding to epithelial bands of left and right dental fields, but also a subfield for symphyseal teeth. Then, intense shh expression is restricted to loci coincident with a temporal series of teeth in iterative jaw positions. The developmental expression of shh reveals previously undetected timing within epithelial stages of tooth formation. Each locus at alternate, even then odd, jaw positions establishes precise sequential timing for successive replacement within each tooth family. Shh appears first in the central cusp, iteratively along the jaw, then reiteratively within each tooth for secondary cusps. This progressive, sequential restriction of shh is shared by toothed gnathostomes and conserved through 500 million years of evolution.
Keywords: catshark, sonic hedgehog, dentition development, tooth patterning, evolution dentition
1. Introduction
Although developmental studies of the mouse dentition have provided most of the molecular data pertaining to the process of tooth development, the sequential addition of new teeth throughout life is missing in this species. Mice have a highly specialized dentition with an extensive diastema disrupting the iterative series of teeth along the jaw, as a normal part of a general vertebrate dentition. Furthermore, most mammals have only one replacement set for the anterior teeth alone and may have sacrificed repetitive replacement tooth initiation for complex molar morphology and occlusion (Jarvinen et al. 2006). In these two respects, the mouse cannot show how genes for tooth development will pattern the entire process for a dentition with replacement teeth for life.
An iterative, close-spaced series of teeth along the jaw is found in the catshark dentition. Moreover, regular tooth replacement is repeated in the adult shark at timed intervals for each tooth along the jaw. This developmental process occurs throughout life and is a characteristic of the apex chondrichthyan predators. Also, each tooth always has multiple cusps and is regularly replaced after every 18–38 days during the life of a shark (Reif et al. 1978). Control of this replacement tooth pattern is not a random process dependent on simple growth parameters and space availability. Eruption time differentials are regulated rostrocaudally along the jaw, as is the cusp pattern, to ensure an identical replacement tooth shape for each position and a precise and regulated process for the dentition (Reif et al. 1978; Smith 2003). This means that the first series of teeth is spaced along the jaw and timed to form at regular intervals with two series, even and odd positions. Also, the replacement teeth for each jaw position develop at sequential times (as seen in the data for the whole developing juvenile dentition in the electronic supplementary material). These times are unique for each tooth in a periodic way both along the jaw and within each family. Morphological asymmetries for left and right start either side of the symphyseal tooth family and can include those within the left and right parasymphyseal tooth sets (see the electronic supplementary material). Moreover, as relative cusp sizes gradually change along the jaw, this can alter shape between whole families. In the catshark, this is a minimal shape change, but, in Heterodontus portusjacksoni, there is a large difference in size and shape between the anterior rasping teeth and the posterior crushing molariform ones.
Positional cues in embryology have been suggested (Reif 1984) to account for the serial repetition of teeth along the jaw margins. Also, their restriction to this oral field along the axis of the jaws may be ascribed to the position of an embryonic border between ectoderm and endoderm. Early in the evolution of dentitions from jawless vertebrates, only the endoderm was endowed with the patterning potential to make tooth sets (Smith 2003). The molecular developmental basis of this restriction of the dentate field, with a mechanism to iteratively create teeth of consistent shape and graded size, has, however, not been addressed.
To gain insights into both how the serial repetition of teeth emerges in S. canicula (spotted catshark) and the sequential production of cusps, we have analysed sonic hedgehog (shh) expression profile before and during this process. Shh is perhaps the most standard marker of mammalian tooth development and it is also expressed in osteichthyan teeth (Fraser et al. 2004). Shh is expressed in the epithelium at the onset of dentition patterning in the mouse and is restricted to the first overt epithelial thickenings at tooth positions (Dassule & McMahon 1998; Cobourne et al. 2004). The Shh protein is secreted by the epithelium and then signals to the underlying mesenchyme (Hardcastle et al. 1998). Control of tooth position involves a combination of sonic signalling at initiation sites and antagonism in edentulous regions such as the mouse diastema (Cobourne et al. 2004). Shh is also part of a molecular pre-pattern for the serial induction of sequential cusps on the mouse molar, predicting the cusp topography more than a day in advance in muroid rodents (Jernvall et al. 2000). Importantly, we have previously demonstrated that key aspects of shh expression are conserved in a basal osteichthyan (Fraser et al. 2004, 2006a,b). In the rainbow trout Oncorhynchus mykiss, shh expression is restricted to the dental epithelium, reciprocally linked with Ombmp4 expression in the papillary mesenchyme, and both are focused at high levels at the sites of tooth germ formation. In osteichthyans, the early field of restricted expression of shh is as one odontogenic band (OB) for each dentate bone, both marginal and palatal (Fraser et al. 2004). Recently, two studies have extended our knowledge of the role of shh in initiation and patterning of the snake dentition (Buchtova et al. 2008; Vonk et al. 2008). These studies have also visualized the OBs as restricted regions of shh expression, separate for each toothed bone, and related this to the determination of subsequent development of the dental lamina and individual tooth germs.
Our results give us insights into the process of patterning the dentition in a chondrichthyan for the first time. Thus, by comparison of the patterning process with trout, snake and mouse, the potentials for conservation over 500 million years of one gene in a common regulatory pathway for all gnathostome teeth can be proposed. We find that temporal and spatial differences in shh expression clearly relate to the order of appearance and spacing of teeth in the catshark. Furthermore, the production of cusps is marked by intense expression of shh in a sequential pattern for each tooth along the tooth row, for central cusps and then accessory ones. Thus, shh expression profiling as a way of marking embryonic tooth-forming epithelium (odontogenic ability) provides information on progressive pattern formation of the dentition, especially for timing and location of tooth loci, and for timing and position of tooth cusps. This gene profile is an effective way of visualizing the pattern formation in chondrichthyans at the basal node on the phylogeny for crown gnathostomes. Here, they represent the most divergent extant form; this will allow comparison with patterning processes for the dentition in all osteichthyans, including reptiles and mammals.
2. Material and methods
Scyliorhinus canicula embryos were taken from the egg cases, anaesthetized (MS222), and approximately 10 specimens from each stage (30–33; Ballard et al. 1993) were processed for in situ hybridization with a probe for Scshh (S. canicula sonic hedgehog; Tanaka et al. 2002). DIG-labelled antisense probes were synthesized according to the manufacturer's instructions (Roche). Heads were fixed in 4 per cent paraformaldehyde (PFA) in phosphate buffered saline (PBS), lower and upper jaws sub-dissected and left overnight at 4°C, then dehydrated through a series of methanol solutions at room temperature and immersed in 100 per cent methanol at −20°C overnight. Rehydration was through a graded series of methanol in PBS 1 per cent Tween-20 (PBT) and followed by incubation in 6 per cent hydrogen peroxide for 60 min at room temperature while rocking. Two 5 min washes in PBT preceded incubation in detergent mix (1% IGEPAL, 1% SDS, 0.5% deoxycholate, 50 mM Tris–HCl (pH 8) 1 mM EDTA and 150 mM NaCl), three times, each for 30 min. Embryos were then washed in PBT twice followed by an incubation in proteinase K (10 μg ml−1), the length of which was determined by the Scyliorhinus growth stage: above stage 30, 25 min; below stage 30, 10 min. Post-fixation was in 0.1 per cent glutaraldehyde, 1 per cent Tween-20 in 4 per cent PFA/PBS for 20 min, followed by two washes in PBT. Hybridization, post-hybridization washes, antibody incubation, washes and colour reaction steps are all according to Myat et al. (1996).
Sections were cut paracoronally or labiolingually through the jaw after whole-mount in situ hybridization, either as 7 μm plastic (processed using glycol methacrylate, Technovit 8100 System, Heraeus Kulzer GmbH, Germany), counterstained with nuclear fast red, or 50 μm gelatin embedded without staining (20% Sigma G9382 Type B). Photomacrographs of lower jaw whole-mount in situ hybridization were taken on a Wild M3Z microscope, and photomicrographs were taken on a Zeiss Photomicroscope III, all with a Nikon Coolpix 990 digital camera and MDC relay lens using Nomarski DIC optics.
3. Results
(a) Spatio-temporal expression of Scshh through morphogenesis of the dentition
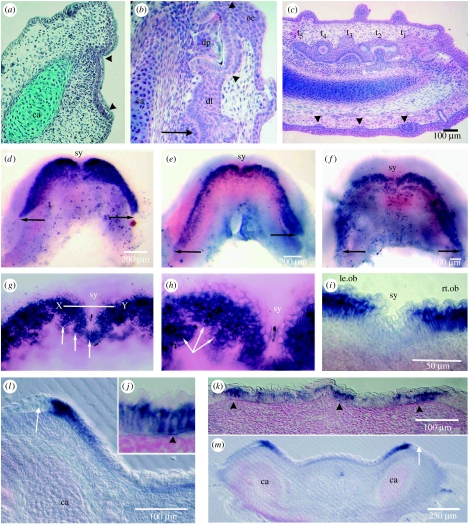
The histological stage, when a thickening of the oral epithelium along the jaw represents the primary OB (arrowheads, figure 1a), precedes any epithelial invagination to form the dental lamina (dl, figure 1b; see also Smith et al. 2008). Exclusively, the tooth germs will form on this infolded epithelium (t1–t5, figure 1c) to form two differently timed series of teeth for alternate even and odd jaw positions (Reif 1980, 1984; for a review, see Smith 2003; also see the electronic supplementary material, figs a, b). By contrast, skin denticles (similar morphogenetic units) form much later and entirely superficially in the epithelium without any epithelial invagination (arrowheads, figure 1c). Within each whole embryo stage (stages 30–32, body lengths 7.9–47.6 mm; Ballard et al. 1993), there are teeth at different stages of development dependent on the individual timing of initiation for each one at its jaw position in the dentition (Reif 1980). From the start of the emergence of the dentition, the changes in development can best be described as sequential ones (stages 1–4). Each one is distinguished by specific distribution of restricted intensity of gene expression, correlated with histology. For instance, the broad histological odontogenic field includes all of the thickened epithelium (arrowheads, figure 1a) and, at stage 1, it is represented by the restricted but broad zones of intense shh expression in lower jaw whole mounts in oral view (figure 1d). At stage 2 (figure 1e,f), shh becomes restricted further to narrower but elongated OBs at the lateral border of the broad field of expression of stage 1. At higher resolution, the rostral regions of the OBs are arranged as clusters of shh-positive epithelial cells (arrows, figure 1g,h).
Figure 1.
Scshh expression related to early pattern development of the lower jaw dentition. (a) Histology with Mallory's trichrome stain of a transverse section through the lower jaw before tooth formation, with a thickened basal layer of epithelium forming the primary OB (arrowheads) associated with a condensation of ectomesenchyme cells. All are located close to the depression in the jaw cartilage (ca) where the tooth families will be situated. (b) H&E section through the dental lamina (dl) with forming tooth germs deep into the oral epithelial surface (oe), alongside the jaw cartilage, same orientation as in (a). This shows one tooth germ with cusp tissue (arrowheads) below the differentiated cells of the inner dental epithelium around a dental papilla (dp). This is the first tooth of the family; deeper on the dental lamina is the early tooth germ of a sequential, alternate series tooth (arrow). (c) H&E of coronal section, right side with extensive dental lamina and five tooth germs (t1–t5) of both even and odd jaw positions (see the electronic supplementary material, fig. a). Tooth germ stages show the position of forming cusp tissue (t1) in the first tooth germ, to the stage of incipient tooth germ (t5). All form on the dental lamina in contrast to the later-developing, superficial dental papillae of skin denticles (arrowheads). (d–h) Oral surface views of whole-mount in situ-hybridized ssh expression in a stage 30–31 series. All show intense expression localization at the oral–aboral boundary to form the primary odontogenic field and later OB. The characteristic cupid's bow shape is at the earliest time with labiolingual intensity gradient of ssh expression in the epithelium representing the primary dental field (d). (e,f) A later stage of odontogenic field expression, equivalent to the OB in (a). There is a posterior extension of the OB (right arrow) and reduction to a thinner intense zone at the outer margin; the difference between the left and right sides may be due to an asymmetric timing for the downregulation of shh. Weaker expression of an inner parallel but defined band may account for the position of the later development of the lingual side of the dental lamina (figure 2l,m). At the stage in (d), there is a gap in the high-intensity dental field expression at the symphysis (sy). The next stage (e–h) shown in close up (g,h), where a midline joining of the shh expression bands forms into groups of cells making the symphyseal subfield (white arrows) in the jaw midline. The line X–Y is shown in section (i) with a distinct absence of expression in the epithelial cells in the midline between the left and right OBs (le.ob and rt.ob). (g,h) Increased magnifications of (e,f) stage OB, where expression is localized to clusters of epithelial cells in the anterior region to form the putative tooth family clones (white arrow); line X–Y is the field of (h,i). (i–k) Sections cut at 7 μm through the stage in (e,f) confirm the basal epithelial location of expression (arrowhead). (l,m) Sections cut at 50 μm through the posterior region of the stage in (e,f) show restriction of intense expression to part of epithelial OB in (a), with sharp junction at the aboral–oral boundary (arrow) located immediately above the jaw cartilage (ca), where a dental lamina will develop before any tooth germs form (figure 3a).
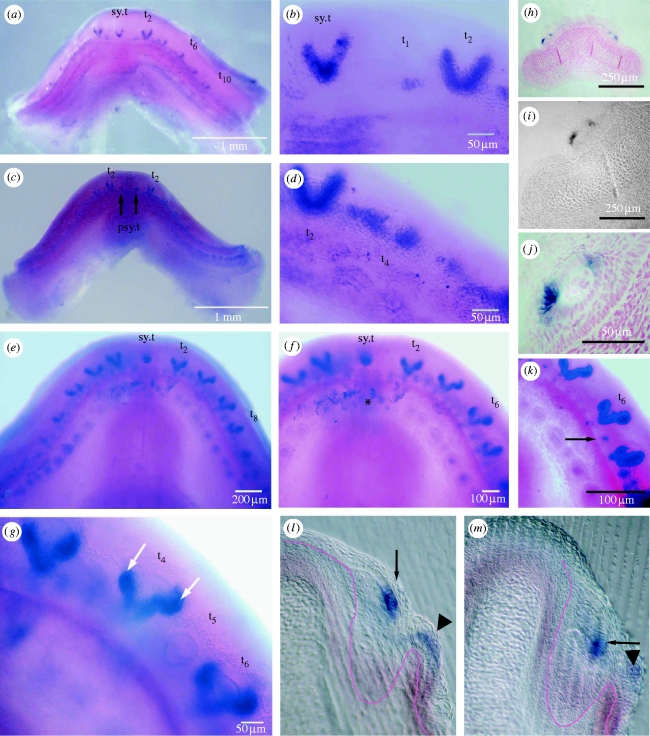
The subsequent infolding of the oral epithelium along the arc of the jaw creates the dental lamina on which the tooth positions are successively created from rostral to caudal and simultaneously left and right. Stage 3 is when induction of the first cusp position in each tooth is identified on the dental lamina (arrow, figures 2l and 3b) and the OB expression is downregulated. At regular intervals, shh expression forms small loci focused at the early tooth positions (t6 and t4, figure 2a,d, respectively), but is later upregulated as a cone-shaped region of expression restricted to the inner dental epithelium in the shape of the first central cusp (figure 2a–d). Progressively, at stage 4, two further accessory cusp positions are first distinguished clearly by cup-shaped intense expression loci as prominent sites in each tooth germ, lateral and basal to the central cusp (figure 2e–g; arrows in figure 2g). As these secondary cusps develop by folding of the dental epithelium (t2, figure 1c), shh expression locates there to the inner dental epithelium. Cusp formation follows with matrix production at the cusp tips (arrowheads, figure 1b); each mineralized cusp can be observed to develop separately before joining to each other and later to the base (see the electronic supplementary material, figs a, b). The regulation of shh expression within these stages is reflected by changes in both the spatial distribution of expression within the epithelium and the levels of expression.
Figure 2.
Scshh expression restricted to focal loci coincident with tooth and cusp positions. (a–g,k) Oral view of whole-mount in situ hybridization of shh, embryo stages 32 and 33. (h–j) Sections cut at 7 μm through specimen (stage 32) in (a) with nuclear fast red stain, at progressive magnification to show that shh expression in teeth at either side is located at the formative end of the inner dental epithelium in both sides of the central cusp. (l,m) Sections cut at 50 μm in the labiolingual plane through specimen in (c). (a–d) Stage 32 with localized shh expression, intense and restricted to the primary cusps of the most anterior tooth positions, symphyseal tooth (sy.t) and first left and right (t2) with up to five others. (b,d) Higher magnification views in which the more posterior teeth show less extensive expression (t4) than the earlier tooth (t2) and the alternate number tooth positions are at the earliest tooth germ stage (t1 in (b)), whereas the more advanced teeth (sy.t, t2) show expression surrounding the cusp shape and present in the inner dental epithelial cells. In (c), there is no symphyseal tooth expression but two sites of gene expression locate to the positions of parasymphyseal teeth (arrows, psy.t). (e,f) Stage 33 in contrast to the jaw in (c) has an intense expression locus for the symphyseal tooth (sy.t) and faint expression for the parasymphyseal tooth germs (all parts of the symphyseal tooth field; see the electronic supplementary material, figure a,b). Teeth of the even series positions (t2–t8) have marked intense expression bilaterally to the central cusp where the secondary cusps will form. (g,k) Higher magnification views of stage 33 tooth germs to emphasize the intense expression associated with the secondary cusps (white arrows). In the position of the two secondary cusps, the intensity and cup shape of the dental epithelium is distinct ((g) t4, t6, white arrows). (k) When contrast is enhanced the alternate tooth germ position to one side of t6 (black arrow) shows as real positive small locus; in (g), this tooth (t5) is seen as a small cone-shaped tooth germ. In (e,f), the loci of weak expression form as an inner row on the lingual fold, as seen in sections ((l,m) arrowhead; left arrow, figure 3b). (l,m) Sections through specimen in (c) in the posterior region with infolding of the dental lamina on which the position of the first germ in the tooth family ((l) arrow) shows strong localized, intense expression. Also in a tooth germ at a later stage of development, expression is in the epithelium at the cusp tip where morphogenesis of the first cusp ((m) arrow) has started.
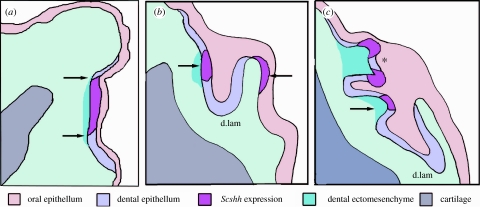
Figure 3.
Schematic to show gene expression as sequential stages of odontogenesis. These are derived from composite information from serial 7 μm sections of whole-mount in situ-hybridized shh expression at several tooth sites along the jaw and from 50 μm gelatin sections where a through-focus series can be observed. Information is also obtained from a comparison with routine histology as in figure 1a–c, where secondary cusps appear and new tooth germs form on the deep extension of the dental lamina; each is associated with ectomesenchyme as dental papillae. (a) Restriction of gene expression to the primary OB is in association with condensations of ectomesenchyme cells (arrows). (b) Focused intense shh expression is restricted to the epithelial thickening for a tooth germ (right arrow) on the epithelial dental lamina (d.lam) associated with papilla ectomesenchyme. This occurs after the oral epithelium has infolded: no expression is seen in the oral epithelium, except very faint expression localized to the lingual epithelium (left arrow) where the lamina epithelium is reflected onto the oral surface but there is no condensed ectomesenchyme. (c) The first tooth is at the morphogenesis stage with intense shh activity (asterisk) in the two accessory cusps; below in the alternate series tooth germ intense shh activity locates to the first cusp position (right arrow).
(i) Tooth region restriction
The youngest lower jaw at stage 1 is observed as a cupid's bow of very strong expression either side of the midline symphysis and clearly defined at the posterior limit of the dentition (arrows, figure 1d), concentration reducing slightly in medial–lingual directions. This primary OB of a broad field of shh expression at stage 1 is restricted to the oral surface in the arc of the jaw where teeth form. There is a distinct symphyseal gap in stage 1 between expression in left and right odontogenic fields (sy, figure 1d); as is emphasized in the section through a region anterior to the symphyseal field (figure 1i). At stage 2, with growth of the jaw, the expression domain relocates as a narrower intense outer band of shh leaving an inner weaker one (figure 1e,f). The outermost OB is bilaterally symmetrical but now linked in the midline by a new territory defined by shh expression joining the left and right bands (figure 1g,h). This we interpret as a sub-region of the dental field, as observed from phenotypic morphology of the whole dentition; it forms the smaller, reduced cusp number of the symphyseal tooth families, with only three cusps as opposed to five (see the electronic supplementary material, figs a, b). The symphyseal families of teeth, one in the midline and left and right parasymphyseal ones, exhibit some variation in their arrangement in adult jaws. As part of stage 2, the more restricted regions of expression correspond with groups of clustered epithelial cells (arrows, figure 1g,h), which we interpret as those now committed to form tooth families at each jaw position. These will progress to form the first teeth on the intucking of the epithelium to provide all their subsequent replacements from a functional dental lamina. The shh-negative regions between the positive cell clusters (figure 1g,h) probably demarcate a precise spacing mechanism that will permit the organization of evenly spaced tooth family units. They represent zones of inhibition (ZOI) of a segmental pattern for which the mechanism is not investigated here.
(ii) Tooth cusp development
Variation in the shape and intensity of gene expression is restricted to tooth loci in the first dentition at stages 3 and 4 (figure 2a–g). This precise restriction of shh to dental epithelial cells surrounding a soft tissue papilla can be related to the first cusp position and then to cusp shape. These tooth germs are in the rostral-most jaw positions of the developing dentition (sy.t, t2, figure 2b,d) in stage 32 embryos. This outline of the central cusp is present in the symphyseal tooth and first teeth either side (left and right in the even positions; t2–t8, figure 2a–e). More posterior and odd tooth positions have expression loci that are less extensive and confined to the pre-cuspal region (t1, t4, figure 2b,d). Expression may vary in timing and shape for the symphyseal tooth by not expressing shh and with weak expression in adjacent parasymphyseal tooth positions (arrows, figure 2c). This probably reflects variation in the phenotypic symphyseal tooth arrangement among individuals, but requires more extensive observations to interpret correctly.
In embryos at stage 33, at dentition stage 4, there are five expression sites at both left and right sides in the shape of primary cusps (figure 2e,f) spaced evenly along the jaw margin. The first four jaw positions, left and right, show that each tooth has strong expression in two extra cup-shaped groups of cells, in the positions either side of the central cusp, where the second and third cusps form in the tooth germ (figure 2e–g,k, arrows in figure 2g). In jaw positions between these germs, at sites where the alternate numbered teeth in the jaw form, there is only very faint expression at tooth sites (t5, figure 2g). This is shown enhanced as a focal spot with extreme contrast adjustment of the image (arrow, figure 2k). The outline of the cusp shape at tooth position 5 (t5) can be seen in figure 2g.
(iii) Gene expression in sections of whole-mount in situ hybridization
shh expression was restricted to the oral surface in basal, columnar epithelial cells at stage 1 as a confirmation of that observed by optical sectioning in the whole jaw mounts (figure 1i–k). At stage 2, the earliest expression is still strong within the basal oral epithelial cells (figure 1l,m) and restricted to the two fields of the left and right jaws. There is an expression gap at the symphysis in the most anterior sections (sy, figure 1i) cut through this region (line X–Y, figure 1g). In more caudal sections, both left and right bands are distinct and more restricted (figure 1l,m). These expression zones coincide with the primary OB of routine histology (figure 1a). shh expression is located in the tall basal cells of this oral epithelium at their basal ends, as distinguished in the 7 μm sections (arrowhead, figure 1j). There is a sharp boundary where shh expression is absent from epithelial cells at the aboral–oral junction, and this region of the epithelium, or ZOI, is one in which an assumed antagonist operates to restrict shh expression (arrow, figure 1l,m). The level of gene expression is strongest in the cells adjacent to a linear array of presumed odontogenic ectomesenchyme. This is prior to the appearance of overt tooth germs and any epithelial folding for a dental lamina.
At tooth initiation (stage 3) expression is localized to the inner dental epithelium of the tooth germ site at the start of the infolding for the dental lamina (arrow, figure 2l) and then to these cells as the primary cusp shape forms (arrow, figure 2m). Only the inner dental epithelium of the tooth germ strongly expresses shh, first in cells around the primary cusp at their formative ends (stage 3, figure 2h–j) and then in the secondary (accessory) cusps. This can be compared with hard tissue formation in the histology of cusp development (arrowheads, figure 1b). In all of these stages, none appears in the superficial epithelium, and none in the surrounding dental lamina cells, neither the outer dental epithelium nor the intermediate cells (dl, figure 1b; figure 2h–j,l,m).
4. Discussion
We have documented, for the first time, in a chondrichthyan with continuous and extensive tooth renewal, embryonic timing of a tooth induction pattern in the dentition and individual cusp development through an analysis of shh expression. We show that shh expressing epithelium co-locates with the known regions of tooth germ formation. shh expression is localized specifically in the oral dental epithelium for iterative events in tooth formation along the jaw margin and within each jaw position. The catshark dentition is identified by the restricted shh expression changing with time at sequential locations along the jaw, where later multiple sets (families) of teeth occur and each position has sequentially timed replacement teeth (for histology, see Smith et al. 2008); for mineralized events, see the electronic supplementary material). These iterative events in sharks provide a predetermined number of tooth sets, in a sequence of tooth initiation established from the midline, and then left and right along the jaw. We predict that the molecular mechanism works through signalling centres in the catshark, comparable with those of the mouse dentition. These shh expression sites correspond in time and space with timing and position of tooth and cusp formation. The fact that cusps form separately, the central one first and then secondary cusps, before joining together and to the tooth base, can be seen in microscopic X-ray computed tomography (microCT) scan studies (see the electronic supplementary material, figs a, b), where mineralized cusps are visualized early in their development. Also, lateral shh expression domains for second and third cusps appear very early, immediately after the primary cusp and before hard tissue formation, marking positions but not relative final heights of cusps. The relative heights of cusps can be seen when mineralized in the images from microCT scan data (see the electronic supplementary material, figs a, b). This difference in timing with later induction of the secondary cusp mineralization suggests further growth in soft tissue shape after upregulation of shh. The spatial temporal sequence in production of cusps in S. canicula of up to five in each tooth can be seen in the microCT scan data (see the electronic supplementary material, figs a, b). Secondary cusps are added iteratively to each initial tooth cusp of the series and in the identical pattern as that identified by localized shh expression. It is proposed that this is a function of sonic hedgehog to facilitate tooth position and shape through timing of secondary cusp positions. This putative function is present from the basal node of jawed vertebrates at which the crown group emerges and is conserved in osteichthyans.
Our results on the expression dynamics of shh during odontogenesis in the catshark reflect those observed in the mouse. In both species, shh is expressed by the epithelium and the expression of this gene coincides with early specification of the dental field, which in mice is referred to as odontogenic epithelium. Then shh expression coincides with each epithelial thickening for tooth positions, and later morphogenetic stages of each tooth germ (Hardcastle et al. 1998). Studies in mice have suggested that Shh is involved in both planar (intra-epithelial) and lateral (epithelial–mesenchymal) signalling during tooth development, and our analysis indicates that this may also be true during odontogenesis in chondrichthyans.
The histological changes as the catshark dentition emerges are described in a comparative study of dental lamina development from that hypothesized for placoderms to all osteichthyans, including teleosts and reptiles (Smith et al. 2008). Smith et al. (2008) propose that there are significant differences in initiation of teeth between chondrichthyans and osteichthyans, which imply lack of homology in the development of a dental lamina. Teleost fishes do not have a continuous dental lamina but sites for each successional tooth form separately from the outer dental epithelium of the older tooth germ (Fraser et al. 2004; Smith et al. 2008; fig. 4). Fraser et al. (2006a) also showed that the replacement teeth use the same cassette of genes. Importantly, they showed that, in osteichthyans, the position of development is different in that neither first nor second teeth form from a dental lamina. Chondrichthyans differ in that all teeth form from a continuous dental lamina, whereas those of teleosts form from oral epithelium and replacements from the prior tooth germ. The dentition in each clade is proposed to have separate evolutionary origins (Smith & Johanson 2003). The spatial expression of shh has also been demonstrated in the snake as initially confined to the OB, prior to dental lamina formation (Buchtova et al. 2008; Vonk et al. 2008). We restrict the term dental lamina to an infolding of the epithelium and not to the superficial field of expression (i.e. the OB) as Buchtova et al. (2008) have done. The boundaries in oral epithelium that separate regions of shh expression are sharply demarcated in the earliest stages and may act to restrict presumptive dental epithelium in the catshark, as also for each dentate bone in the trout (Fraser et al. 2004). This would suggest that, as in the trout, snake and mouse, Shh expression may be actively repressed in the non-oral ectoderm, and the mouse data would suggest that this would probably involve a Wnt gene. Sarkar et al. (2000) showed that Wnt-7b expression is reciprocal to that of Shh in the mouse for all stages of tooth development and that these genes mark the boundaries between cells of different developmental fates. Fraser et al. (2008) have shown that the pattern for Wnt-7b expression is also reciprocal to that of shh in the developing cichlid dentition, suggesting an equivalent role in restricting regions for tooth formation. Using a whole suite of genes known to pattern iterative structures in vertebrates, they have shown that coordinated patterns of gene expression restrict positions in each new tooth row in these multi-rowed iterative dentitions.
Here, we have shown that the timing and restricted location of shh expression in tooth initiation is iterative and coincides with iterative developmental stages known for tooth morphogenesis in the catshark. This is both for the first sites of tooth germs and then for each cusp on the crown as morphogenesis unfolds sequentially at each tooth position. The intense shh expression and precise location in the dental epithelium to classic stages of morphogenesis of mammalian teeth demonstrates that these sites could be acting as signalling centres, in the same way as is known for mammalian molars (Thesleff & Jernvall 1997). Putative cusp position signalling for the first cusp, followed by second and third cusps, has not previously been shown for any non-mammalian vertebrate. As we suggest, this indicates that one gene for tooth patterning is conserved from a basal jawed vertebrate to mammals spanning some 500 million years of evolution. Shh expression was detected in the mouse molar in pre-cusp positions, co-located with several other signals, Bmp2, -4 and -7 (Vaahtokari et al. 1996a,b; Thesleff & Jernvall 1997), as well as Fgf9 (Kettunen & Thesleff 1998) and Wnt-10b (Dassule & McMahon 1998). These sites, identified by histology as enamel knots, were first shown to be sites of gene expression restriction with Fgf-4 coincident with non-dividing cells (Jernvall et al. 1994). Secondary cusp positions were also identified as enamel knots, with the same suite of expressed genes. An obvious future study would be to explore the expression pattern of some of these genes involved in the production of multiple sets of teeth in the catshark. However, a histological structure equivalent to the mammalian enamel knot is not present in the catshark tooth germ (Smith et al. 2008). In a study of molecular signalling in the python and grass snake dentitions, Buchtova et al. (2008) also denied the existence of an exact histological equivalent but did demonstrate high levels of shh transcripts and low cell proliferation in the entire inner epithelium. The expression pattern of shh throughout the inner dental epithelium at the morphogenetic stage in the catshark conforms more to that of the trout (Fraser et al. 2004) and the snake than the mouse. Buchtova et al. (2008) concluded by direct comparison with the mouse that the inner dental epithelium, with other cell layers of the snake tooth germ, could be considered a functional homologue of the mammalian enamel knot. Harris et al. (2006) compared the formation of teeth in the talpid chick mutant with the alligator and demonstrated ‘distinct round foci of shh expression in the tooth anlagen’. Before these studies, the reiterative use of Shh had not been known outside mammals, from tooth field specification to tooth site location and then to tooth cusp positions (Jernvall et al. 2000). A similar study by Fraser et al. (2004), including shh, pitx2 and bmp4 examined for spatio-temporal expression, did demonstrate that, at all tooth germ sites, these genes are used to pattern an evolutionary stable event within osteichthyans, since their divergence at 420 Myr ago.
Smith & Johanson (2003) discussed the origins in the evolution of a dental lamina for gnathostomes, in the context of the exclusively fossil group placoderms. They proposed that, after the evolution of jaws, four independent events occurred to make a dentition pattern, on each of four gnathostomes lineages. Fraser et al. (2004) proposed that teeth form from a modular unit with phenotypic stability conserved at the level of developmental genes. The catshark similarly uses the same modular developmental programme but the dentition is different in the way these modules are patterned on the dental lamina. This is dependent on as yet unknown genetic properties of the dental lamina as all teeth, primary and secondary, form from this continuous epithelial invagination. The putative location for the site that may contain stem cells to regulate timing of new teeth in the catshark is discussed by Smith et al. (2008) and proposed to be the intermediate cells of the dental lamina. At the level of the tooth module, visualized as expression loci for teeth and cusps, expression of one gene used for morphogenetic stages is shared by all clades of jawed vertebrates. However, the way in which these modules are organized, directly from the oral epithelium or a sub-oral epithelial dental lamina, may be derived by independent evolution for the dentition type of each major clade.
Acknowledgments
We thank Cheryll Tickle, University of Bath, for the gift of the Scshh probe; Mikiko Tanaka, Tokyo Institute of Technology, for plasmids and whole embryos hybridized with the shh probe at earlier stages; and Christopher Healy for the production of the microCT scan data in the Department of Craniofacial Development of the Dental Institute at KCL.
Supplementary Material
References
- Ballard W., Mellinger J., Lechenault H. A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish. J. Exp. Zool. 1993;267:318–336. doi:10.1002/jez.1402670309 [Google Scholar]
- Buchtova M., Handrigan G.R., Tucker A.S., Lozanoff S., Town L., Fu K., Diewert V.M., Wicking C., Richman J.M. Initiation and patterning of the snake dentition are dependent on Sonic Hedgehog signaling. Dev. Biol. 2008;319:132–145. doi: 10.1016/j.ydbio.2008.03.004. doi:10.1016/j.ydbio.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Cobourne M.T., Miletich I., Sharpe P.T. Restriction of sonic hedgehog signalling during early tooth development. Development. 2004;131:2875–2885. doi: 10.1242/dev.01163. doi:10.1242/dev.01163 [DOI] [PubMed] [Google Scholar]
- Dassule H.R., McMahon A.P. Analysis of epithelial–mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev. Biol. 1998;202:215–227. doi: 10.1006/dbio.1998.8992. doi:10.1006/dbio.1998.8992 [DOI] [PubMed] [Google Scholar]
- Fraser G.J., Graham A., Smith M.M. Conserved deployment of genes during odontogenesis across osteichthyans. Proc. R. Soc. B. 2004;271:2311–2317. doi: 10.1098/rspb.2004.2878. doi:10.1098/rspb.2004.2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser G.J., Berkovitz B.K., Graham A., Smith M.M. Gene deployment for tooth replacement in the rainbow trout (Oncorhynchus mykiss): a developmental model for evolution of the osteichthyan dentition. Evol. Dev. 2006a;8:446–457. doi: 10.1111/j.1525-142X.2006.00118.x. doi:10.1111/j.1525-142X.2006.00118.x [DOI] [PubMed] [Google Scholar]
- Fraser G.J., Graham A., Smith M.M. Developmental and evolutionary origins of the vertebrate dentition: molecular controls for spatio-temporal organisation of tooth sites in osteichthyans. J. Exp. Zool. B: Mol. Dev. Evol. 2006b;306:183–203. doi: 10.1002/jez.b.21097. doi:10.1002/jez.b.21097 [DOI] [PubMed] [Google Scholar]
- Fraser G.J., Bloomquist R.F., Streelman J.T. A periodic pattern generator for dental diversity. BMC Biol. 2008;6:1–33. doi: 10.1186/1741-7007-6-32. doi:10.1186/1741-7007-6-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle Z., Mo R., Hui C.C., Sharpe P.T. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125:2803–2811. doi: 10.1242/dev.125.15.2803. [DOI] [PubMed] [Google Scholar]
- Harris M.P., Hasso S.M., Ferguson M.W., Fallon J.F. The development of archosaurian first-generation teeth in a chicken mutant. Curr. Biol. 2006;16:371–377. doi: 10.1016/j.cub.2005.12.047. doi:10.1016/j.cub.2005.12.047 [DOI] [PubMed] [Google Scholar]
- Jarvinen E., Salazar-Ciudad I., Birchmeier W., Taketo M.M., Jernvall J., Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc. Natl Acad. Sci. USA. 2006;103:18 627–18 632. doi: 10.1073/pnas.0607289103. doi:10.1073/pnas.0607289103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J., Kettunen P., Karavanova I., Martin L.B., Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int. J. Dev. Biol. 1994;38:463–469. [PubMed] [Google Scholar]
- Jernvall J., Keranen S.V., Thesleff I. Evolutionary modification of development in mammalian teeth: quantifying gene expression patterns and topography. Proc. Natl Acad. Sci. USA. 2000;97:14 444–14 448. doi: 10.1073/pnas.97.26.14444. doi:10.1073/pnas.97.26.14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen P., Thesleff I. Expression and function of FGFs-4, -8, and -9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev. Dyn. 1998;211:256–268. doi: 10.1002/(SICI)1097-0177(199803)211:3<256::AID-AJA7>3.0.CO;2-G. doi:10.1002/(SICI)1097-0177(199803)211:3<256::AID-AJA7>3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- Myat A., Henrique D., Ish-Horowicz D., Lewis J. A chick homologue of Serrate and its relationship with Notch and Delta homologues during central neurogenesis. Dev. Biol. 1996;174:233–247. doi: 10.1006/dbio.1996.0069. doi:10.1006/dbio.1996.0069 [DOI] [PubMed] [Google Scholar]
- Reif W.E. Development of dentition and dermal skeleton in embryonic Scyliorhinus canicula. J. Morphol. 1980;166:275–288. doi: 10.1002/jmor.1051660303. doi:10.1002/jmor.1051660303 [DOI] [PubMed] [Google Scholar]
- Reif W.E. Pattern regulation in shark dentitions. In: Malakinski G., editor. Pattern formation: a primer in developmental biololgy. Macmillan; New York, NY: 1984. pp. 603–621. [Google Scholar]
- Reif W.E., McGill D., Motta P. Tooth replacement rates of the sharks Trikias semifasciata and Ginglymostoma cirratum. Zoll. Jb. Anat. 1978;99:151–156. [Google Scholar]
- Sarkar L., Cobourne M., Naylor S., Smalley M., Dale T., Sharpe P.T. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc. Natl Acad. Sci. USA. 2000;97:4520–4524. doi: 10.1073/pnas.97.9.4520. doi:10.1073/pnas.97.9.4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.M. Vertebrate dentitions at the origin of jaws: when and how pattern evolved. Evol. Dev. 2003;5:394–413. doi: 10.1046/j.1525-142x.2003.03047.x. doi:10.1046/j.1525-142X.2003.03047.x [DOI] [PubMed] [Google Scholar]
- Smith M.M., Johanson Z. Separate evolutionary origins of teeth from evidence in fossil jawed vertebrates. Science. 2003;299:1235–1236. doi: 10.1126/science.1079623. doi:10.1126/science.1079623 [DOI] [PubMed] [Google Scholar]
- Smith M.M., Fraser G.J., Mitsiadis T. Dental lamina as a source of odontogenic stem cells: evolutionary origins and developmental control of tooth generation in gnathostomes. J. Exp. Zool B: Mol. Dev. Evol. 2008;310B:1–21. doi: 10.1002/jez.b.21272. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Münsterberg A., Anderson W.G., Prescott A.R., Hazon N., Tickle C. Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature. 2002;416:527–531. doi: 10.1038/416527a. doi:10.1038/416527a [DOI] [PubMed] [Google Scholar]
- Thesleff I., Jernvall J. The enamel knot: a putative signaling center regulating tooth development. Cold Spring Harb. Symp. Quant. Biol. 1997;62:257–267. [PubMed] [Google Scholar]
- Vaahtokari A., Aberg T., Jernvall J., Keranen S., Thesleff I. The enamel knot as a signaling center in the developing mouse tooth. Mech. Dev. 1996a;54:39–43. doi: 10.1016/0925-4773(95)00459-9. doi:10.1016/0925-4773(95)00459-9 [DOI] [PubMed] [Google Scholar]
- Vaahtokari A., Aberg T., Thesleff I. Apoptosis in the developing tooth: association with an embryonic signaling center and suppression by EGF and FGF-4. Development. 1996b;122:121–129. doi: 10.1242/dev.122.1.121. [DOI] [PubMed] [Google Scholar]
- Vonk F.J., et al. Evolutionary origin and development of snake fangs. Nature. 2008;454:630–633. doi: 10.1038/nature07178. doi:10.1038/nature07178 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.