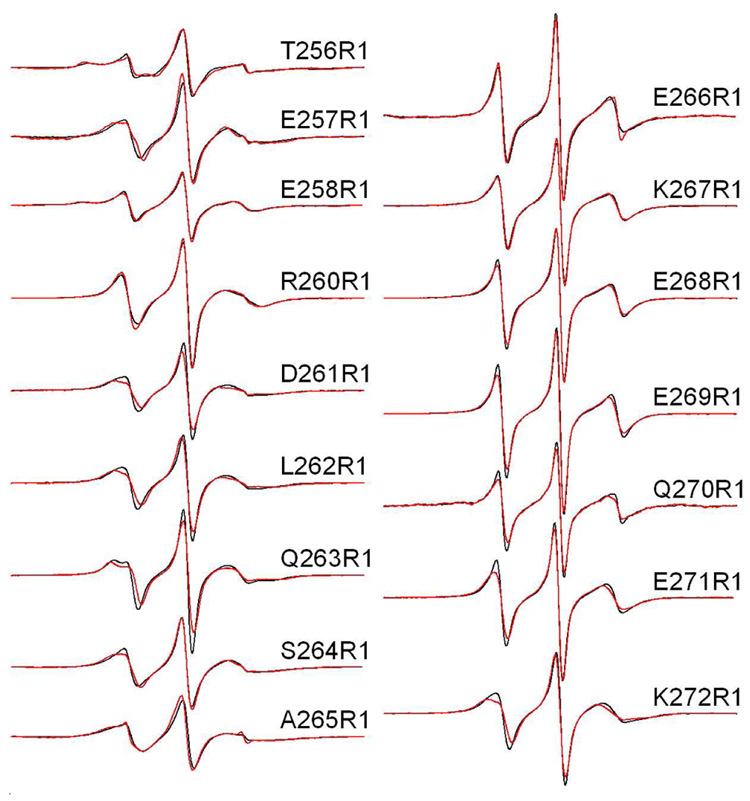
Figure 2.
X-band EPR spectra of single R1 substitutions in C2AB in the presence of Ca2+. Aqueous spectra are shown in black, spectra of C2AB completely bound to POPC:POPS (75:25) vesicles are shown in red. Spin-labeled mutants within the linker encompass residues 264–272 and mutants 256–263 cover the last β-strand in C2A. The spectra are normalized relative to each other, and the relative amplitudes provide an indication of relative nitroxide motion. The spectra are 100 Gauss scans. The spin-labeled protein concentrations in these experiments was 50 µM, with lipid concentrations of approximately 1000 fold higher (50 mM or greater). This lipid concentration is well in excess of that needed to completely bind syt1C2AB as shown previously (11).